
中国水稻科学 ›› 2026, Vol. 40 ›› Issue (1): 37-50.DOI: 10.16819/j.1001-7216.2026.250202
岳轩宇1,2, 谢文亚1,2,3,*( ), 冯志明1,2,3, 陈宗祥1,2,3, 胡珂鸣1,2, 左示敏1,2,3,*(
), 冯志明1,2,3, 陈宗祥1,2,3, 胡珂鸣1,2, 左示敏1,2,3,*( )
)
收稿日期:2025-02-11
修回日期:2025-05-23
出版日期:2026-01-10
发布日期:2026-01-21
通讯作者:
*email:smzuo@yzu.edu.cn;基金资助:
YUE Xuanyu1,2, XIE Wenya1,2,3,*( ), FENG Zhiming1,2,3, CHEN Zongxiang1,2,3, HU Keming1,2, ZUO Shimin1,2,3,*(
), FENG Zhiming1,2,3, CHEN Zongxiang1,2,3, HU Keming1,2, ZUO Shimin1,2,3,*( )
)
Received:2025-02-11
Revised:2025-05-23
Online:2026-01-10
Published:2026-01-21
摘要:
【目的】本研究旨在解析ERF类转录因子OsERF93在水稻抗纹枯病中的功能与机制,为抗病育种提供基因资源。【方法】利用RT-qPCR、水稻原生质体转录激活等方法,分析了OsERF93的表达模式、转录调控功能和亚细胞定位位置;通过构建转基因过表达和敲除株系,研究了OsERF93调控纹枯病抗性的功能,进而比较分析了水杨酸、茉莉酸和乙烯信号相关基因在转基因系接种前后的表达差异,以及超表达转基因系与对照间的农艺性状差异。【结果】OsERF93在水稻叶片和叶鞘组织中高表达,受纹枯病菌侵染诱导表达;其编码蛋白定位于细胞核和细胞膜中,具有转录激活活性;OsERF93过表达显著增强了水稻纹枯病抗性,而敲除则显著减弱抗病性;茉莉酸合成基因OsAOS2以及部分PR基因在OsERF93过表达系和敲除系中分别明显上调和下调表达;OsERF93过表达系的株高和千粒重略低于野生型对照,但每穗粒数显著高于对照,其他性状如分蘖数、一次枝梗数和穗长与对照间无显著差异。【结论】OsERF93通过激活茉莉酸信号途径正调控水稻的纹枯病抗性,为水稻抗病育种提供了一个新的基因资源。
岳轩宇, 谢文亚, 冯志明, 陈宗祥, 胡珂鸣, 左示敏. OsERF93参与调控水稻纹枯病抗性的研究[J]. 中国水稻科学, 2026, 40(1): 37-50.
YUE Xuanyu, XIE Wenya, FENG Zhiming, CHEN Zongxiang, HU Keming, ZUO Shimin. Function of OsERF93 in Regulating Resistance to Sheath Blight in Rice[J]. Chinese Journal OF Rice Science, 2026, 40(1): 37-50.
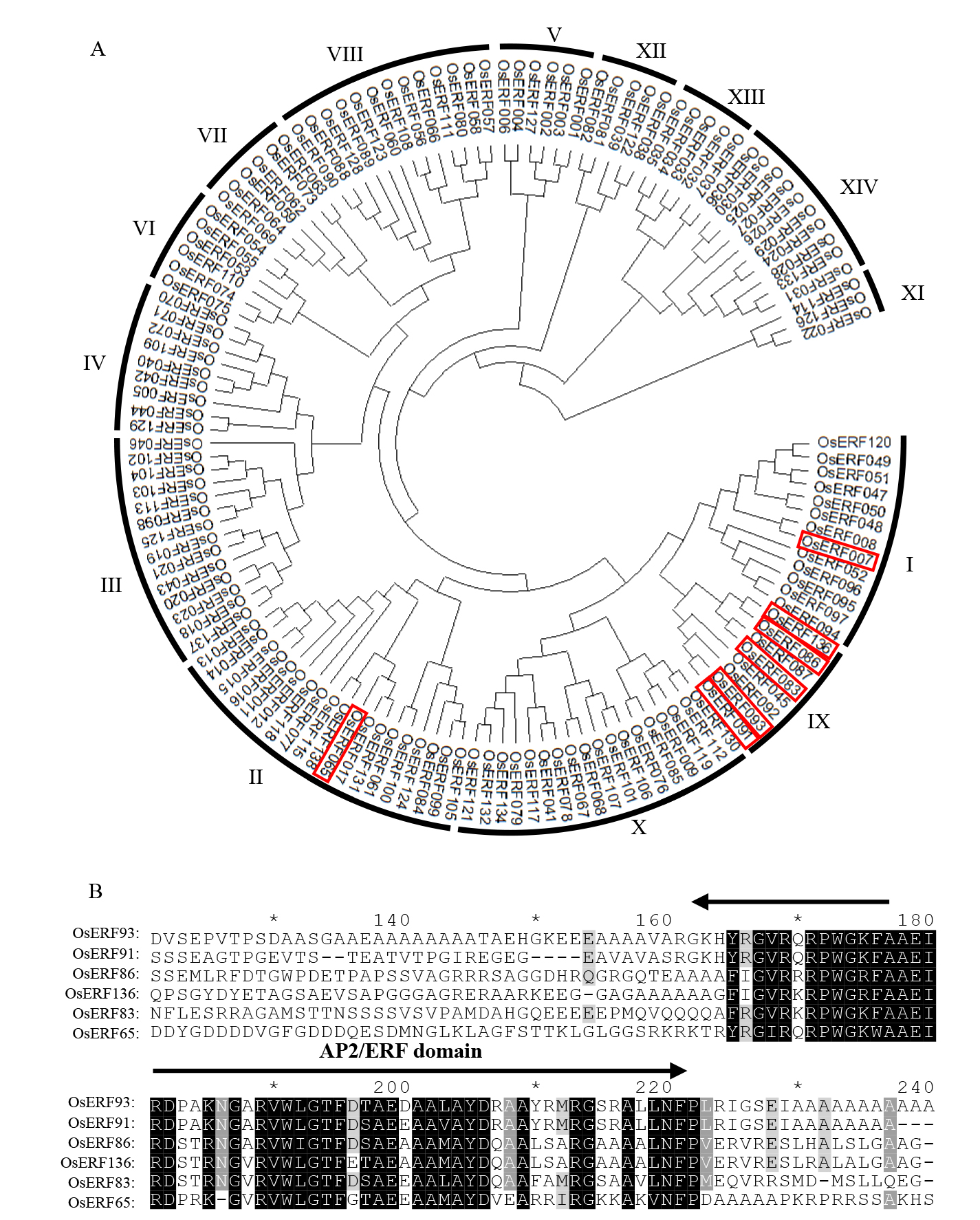
图1 水稻中的OsERF基因家族聚类分析与OsERF93所在亚家族蛋白序列比对 A:ERF基因家族系统发育树分析,红框标注的基因在纹枯病菌接种后的抗感材料中表现出差异性表达;B:参与响应纹枯病诱导的ERF家族基因的相关蛋白序列比对。
Fig. 1. Phylogenetic analysis of rice OsERFs gene family and protein sequence alignment of subfamily containing OsERF93 A, Phylogenetic tree analysis of the ERFs gene family, with the genes highlighted in red boxes showing differential expression in resistant and susceptible materials after inoculation with R. Solani; B, Alignment of protein sequence of ERF family genes involved in sheath blight response.
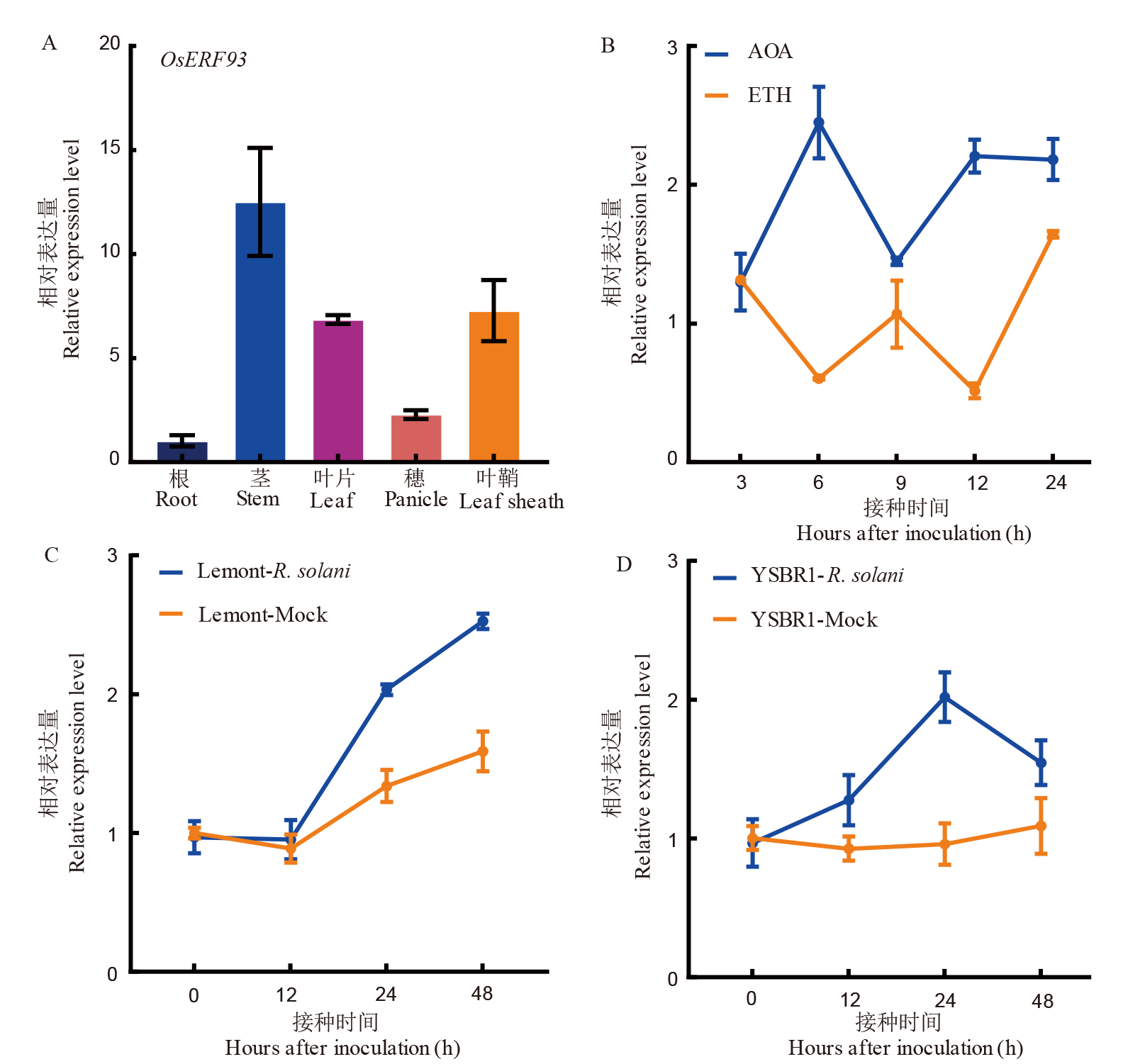
图2 OsERF93诱导表达模式分析 A:OsERF93组织特异性表达;B:OsERF93对ETH和AOA的不同响应;C:感病品种Lemont接种纹枯病菌后相对表达量变化;D:抗病品种YSBR1接种纹枯病后相对表达量变化。图中R. solani为实验组,Mock为对照组。
Fig. 2. Analysis of OsERF93 expression pattern A, Tissue-specific expression of OsERF93; B, Differential response of OsERF93 to ETH (Ethylene) and AOA (Aminocyclopropane-1-carboxylic acid); C, Relative expression levels of the susceptible variety Lemont after inoculation with sheath blight pathogen; D, Relative expression levels of the resistant variety YSBR1 after inoculation with sheath blight pathogen. In the figures, R. solani represents the treatment group, and Mock represents the control group.
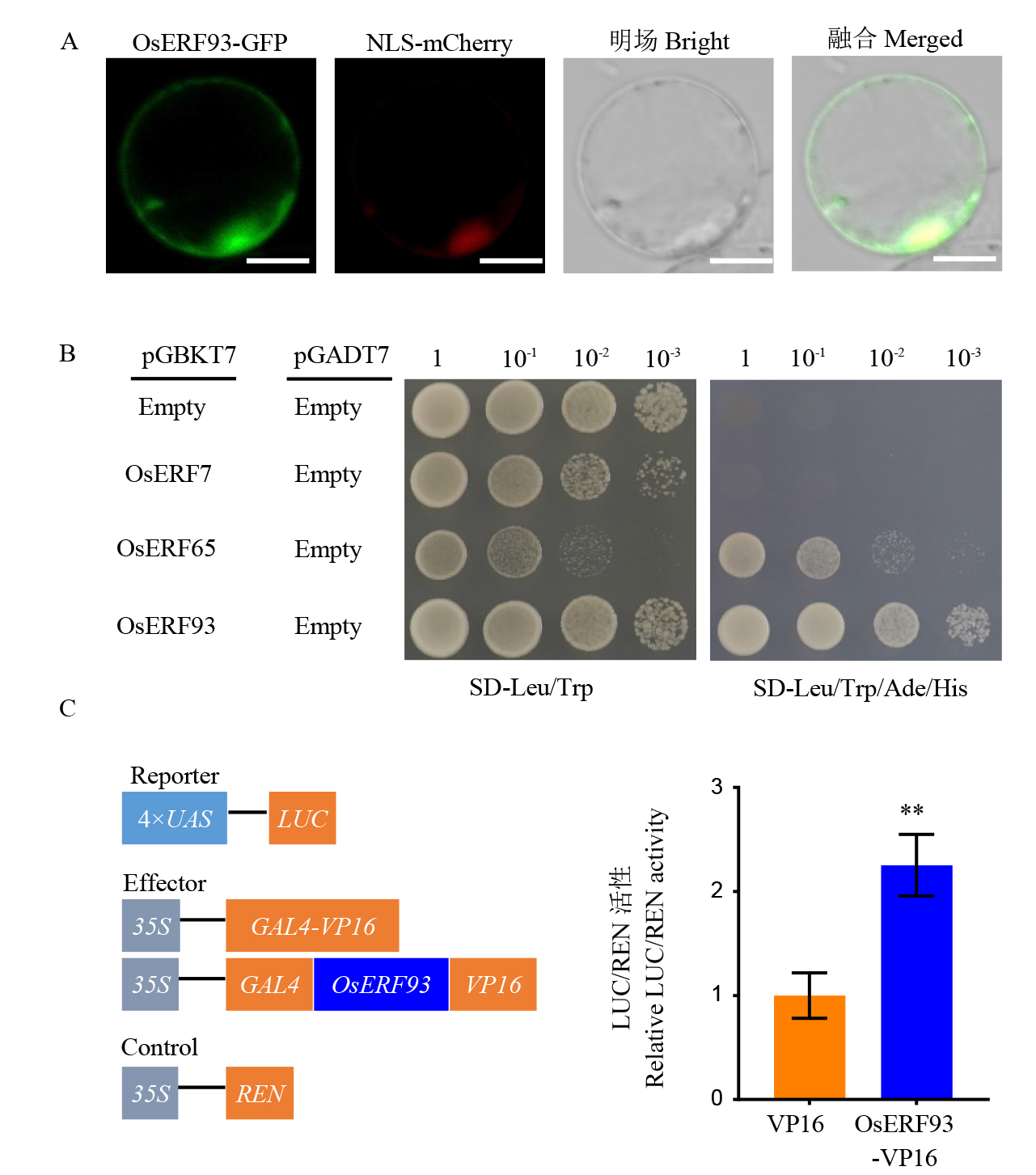
图3 OsERF93转录激活活性与亚细胞定位分析 A:OsERF93在水稻细胞中的亚细胞定位,标尺=10 μm,NLS为细胞核定位信号;B:酵母双杂交实验验证转录激活活性;C:水稻原生质体瞬时转化,VP16 是转录激活对照。**表示由 P< 0.01 的t 检验确定的极显著差异。
Fig. 3. Analysis of transcriptional activation activity and subcellular localization of OsERF93 A, Subcellular localization of OsERF93 in rice cells(bar=10 μm), and NLS denoting the nuclear localization signal; B, Transcriptional activation activity verified by yeast two-hybrid assay; C, Transient transformation of rice protoplasts, with VP16 serving as the transcriptional activation control. ** indicates a highly significant difference as determined by a t-test with P< 0.01.
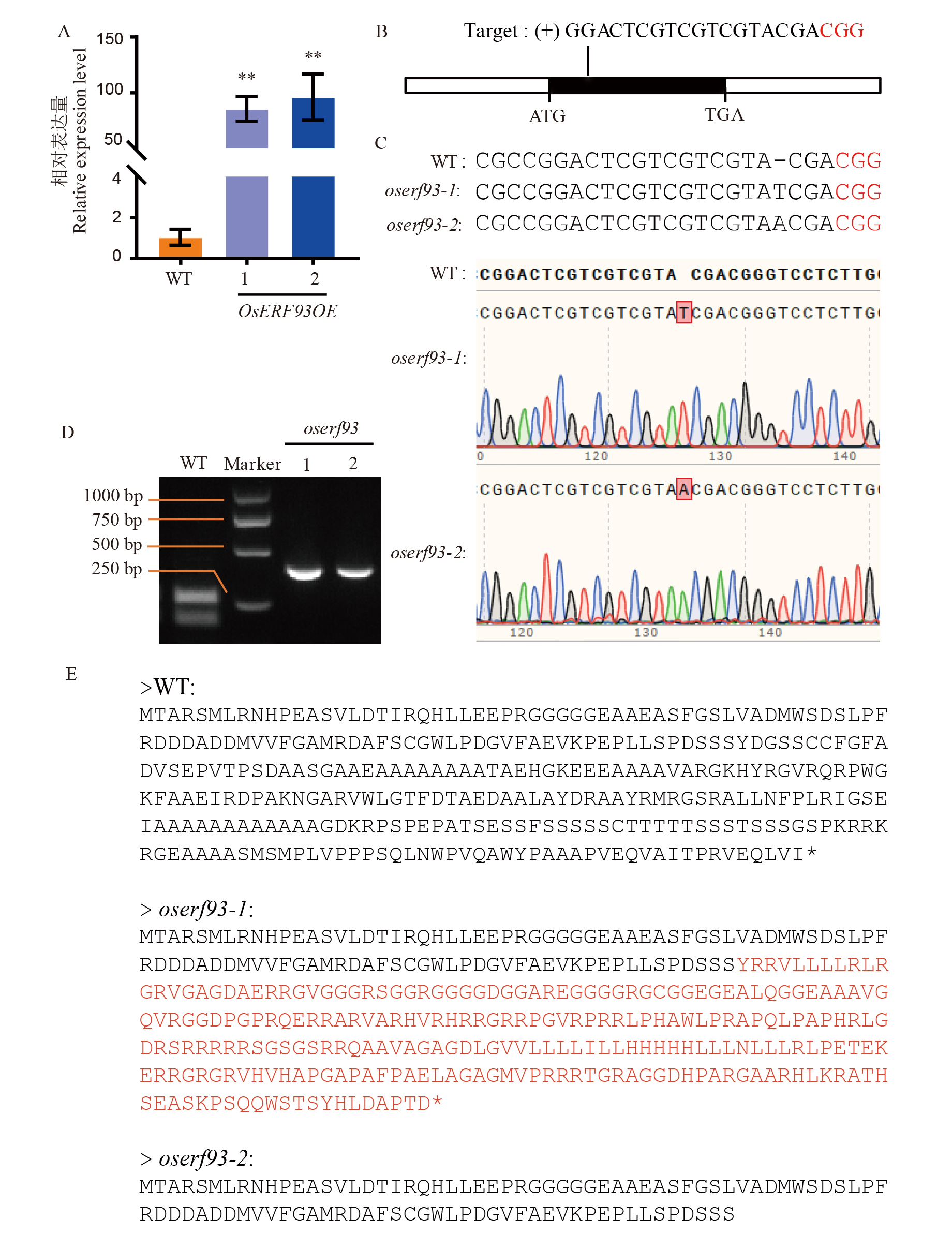
图4 OsERF93基因过表达系和敲除突变体的分子鉴定 A:两个OsERF93过表达株系中OsERF93的转录水平;B:OsERF93基因结构和CRISPR/Cas9靶点示意图;C:鉴定出的两个纯合oserf93突变体与野生型对照的DNA序列比对;D:CAPS标记验证oserf93突变体与野生型,引物序列见附表1,oserf93-1和oserf93-1片段长度为417 bp,野生型被酶切后片段长度分别为248 bp和169 bp;E:野生型及两个敲除系中OsERF93基因编码的氨基酸序列分析。
Fig. 4. Relative expression levels of the OsERF93 overexpression lines A, Transcript levels of OsERF93 in two OsERF93 overexpression lines; B, Schematic diagram of OsERF93 gene structure and CRISPR/Cas9 target sites; C, DNA sequence alignment of the identified homozygous oserf93 mutants with the wild-type control; D, CAPS marker validation of oserf93 mutants and wild-type, with primer sequences listed in Table S1, showing fragment lengths of 417 bp for oserf93-1 and oserf93-2, and the wild-type fragments being 248 bp and 169 bp after enzyme digestion; E, Amino acid sequence analysis of the OsERF93 gene encoded in the wild-type and two knockout lines.
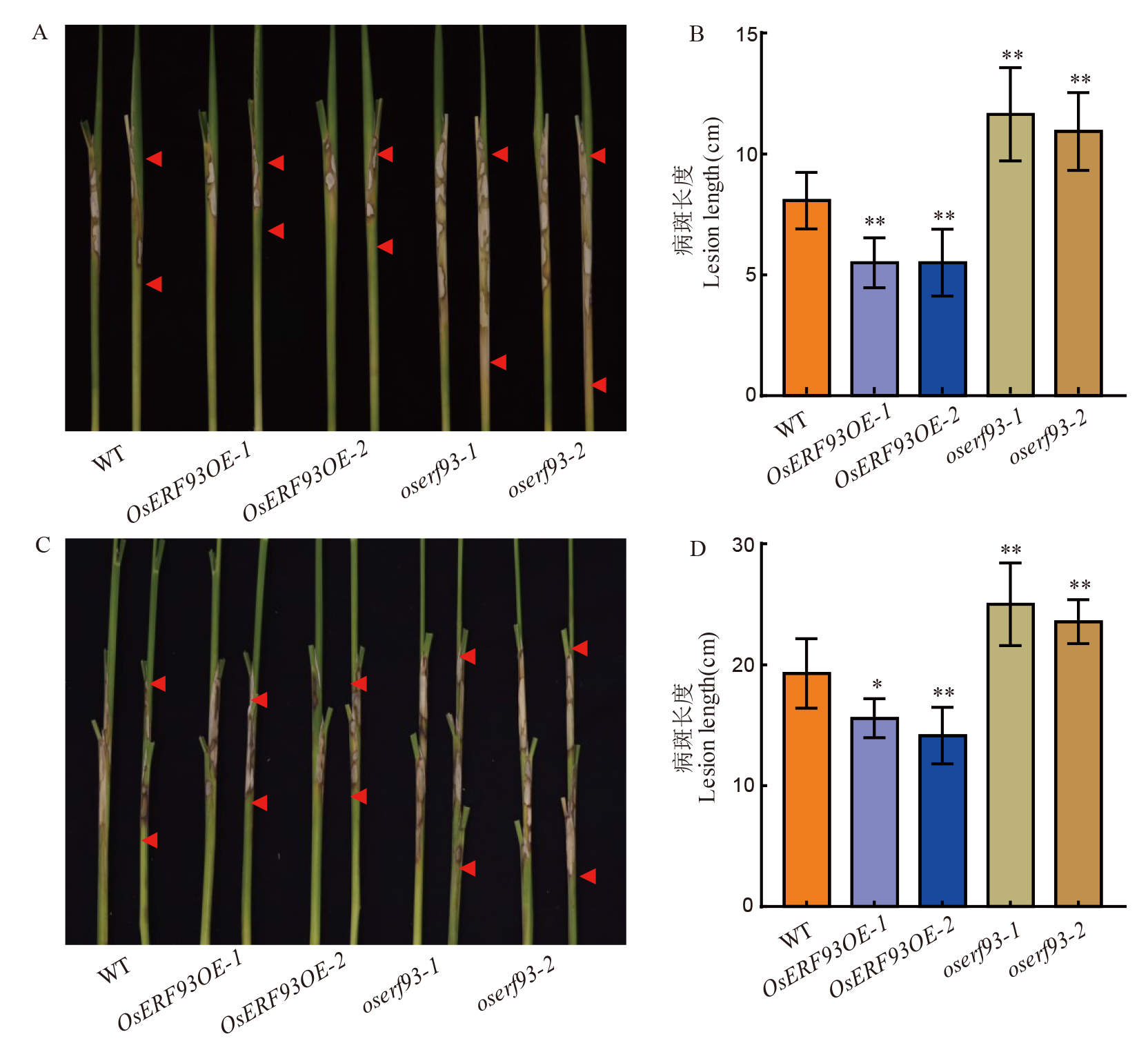
图5 OsERF93超表达和敲除系的纹枯病抗性鉴定 A和B分别为离体茎秆接种鉴定中对照及OsERF93相关转基因系接种后5 d的表型照片和病斑长度统计比较;C和D分别为温室成株期植株接种鉴定中OsERF93相关转基因系接种后14 d的结果和病斑长度。**和*分别表示P< 0.01和P< 0.05 统计显著性差异水平。
Fig. 5. Resistance evaluation of OsERF93 overexpression and knock-out lines to sheath blight disease A and B respectively present the phenotypic photographs and statistical comparisons of lesion lengths at 5 days post-inoculation in the control and OsERF93-related transgenic lines during the in vitro stem inoculation assay; C and D respectively display the phenotypic photographs and statistical comparisons of lesion heights at 14 days post-inoculation in the OsERF93-related transgenic lines during the greenhouse adult plant inoculation assay. ** and * indicate statistical significance levels of P< 0.01 and P< 0.05, respectively.
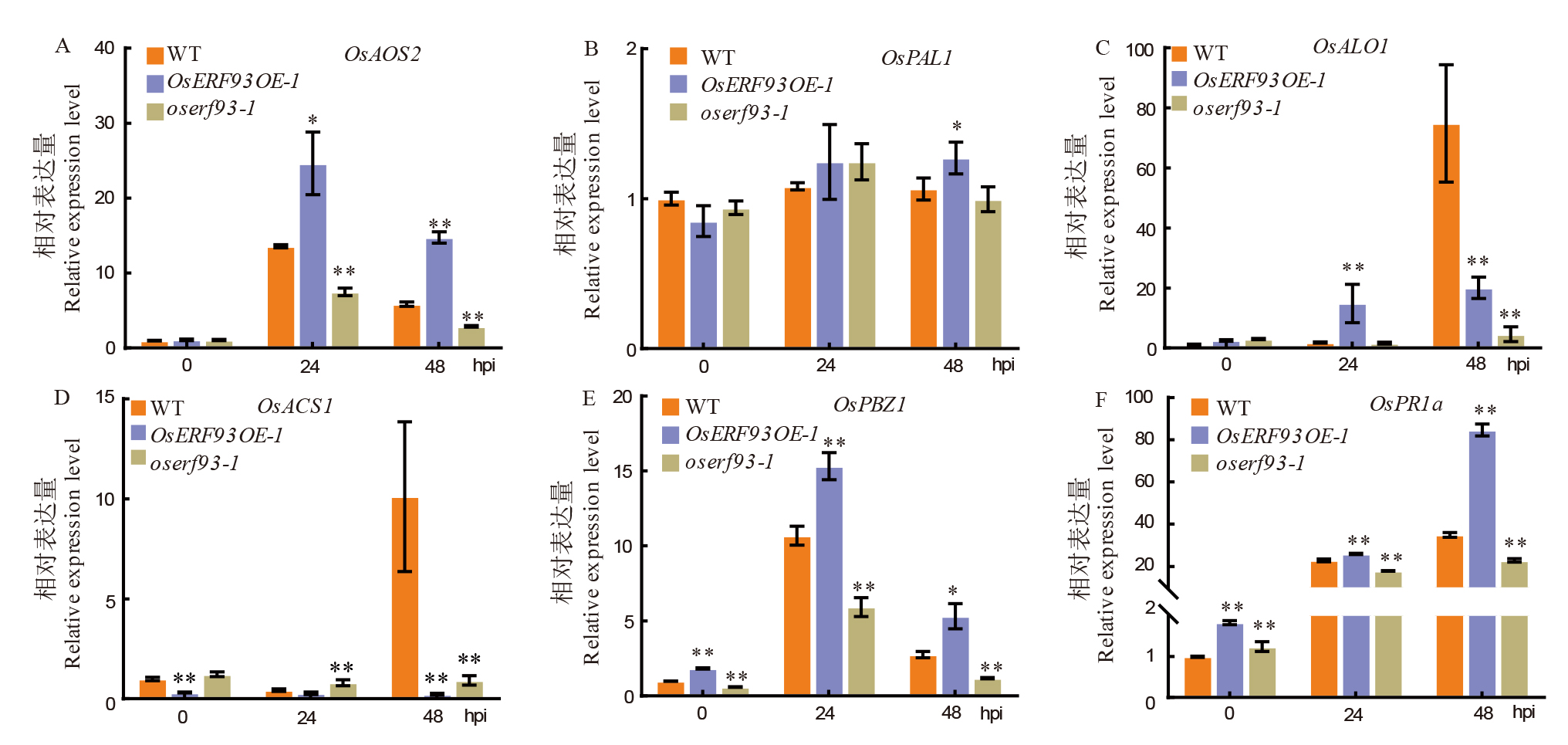
图6 OsERF93超表达和敲除系中部分抗病相关基因在病原菌接种前后的表达变化特征 A和B分别为茉莉酸合成基因OsAOS2、水杨酸途径标志基因OsPAL1在野生型对照及OsERF93相关转基因系接种纹枯病菌后的表达量变化;C和D是乙烯信号相关基因OsACO1和OsACS1在野生型和OsERF93相关转基因系接种纹枯病菌前后的表达量变化;E和F分别为病程相关蛋白基因OsPBZ1和OsPR1a在野生型及OsERF93相关转基因系接种纹枯病菌后的表达量变化。 **和*分别表示P< 0.01和P< 0.05 统计显著性差异水平。
Fig. 6. Expression changes of pathogenesis-related genes in OsERF93 overexpression and knock-out lines after inoculation with sheath blight fungus A and B respectively depict the expression level changes of the jasmonic acid signal related gene OsAOS2 and the salicylic acid pathway marker gene OsPAL1 in the wild-type control and OsERF93-related transgenic lines after inoculation with the sheath blight pathogen; C and D represent the expression changes of ethylene signal related genes OsACO1 and OsACS1 in wild-type and OsERF93-related transgenic lines after inoculation with the sheath blight pathogen; E and F respectively show the expression level changes of the pathogenesis-related protein genes OsPBZ1 and OsPR1a in the wild-type and OsERF93-related transgenic lines after inoculation with the sheath blight pathogen. ** and * denote statistical significance levels of P< 0.01 and P< 0.05, respectively.
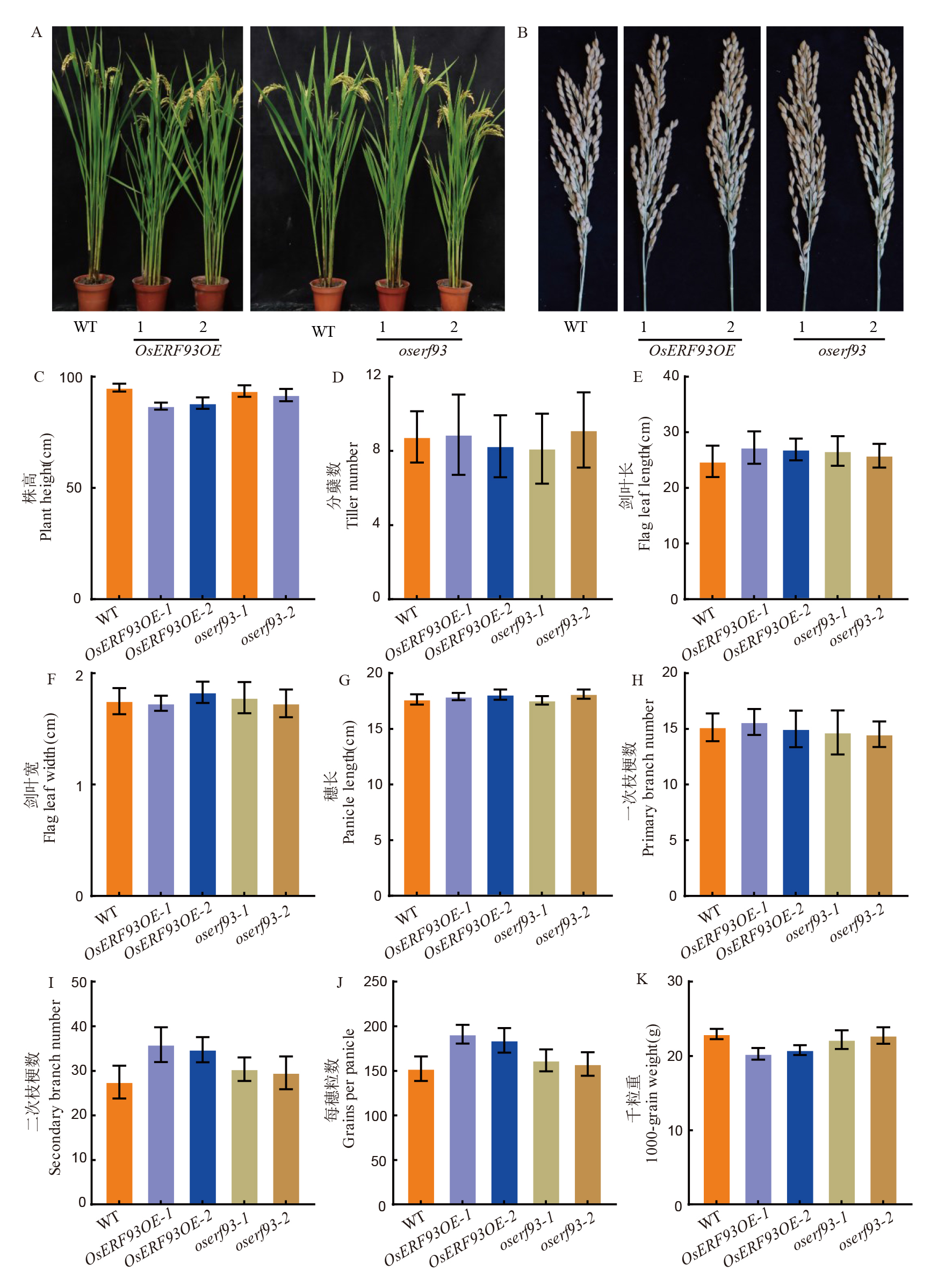
图7 OsERF93相关转基因系及其野生型间的部分农艺性状比较 A和B分别为野生型(WT)、OsERF93过表达系(OsERF93OE-1、OsERF93OE-2)及敲除系(oserf93-1、oserf93-1-2)的株型和穗型照片比较;C~K分别为野生型(WT)、OsERF93过表达系及敲除系在株高(C)、分蘖数(D)、剑叶长(E)、剑叶宽(F)、穗长(G)、一次枝梗数(H)、二次枝梗数(I)、穗粒数(J)和千粒重(K)性状上的差异统计比较;**和*分别表示P< 0.01和P< 0.05 统计显著性差异水平。
Fig. 7. Comparison of some agronomic traits among WT, OsERF93 overexpression and knock-out transgenic lines A and B show comparisons of plant architecture and panicle morphology between the wild-type (WT), OsERF93 overexpression lines (OsERF93OE-1, OsERF93OE-2), and knockout lines (oserf93-1, oserf93-2). C-K present statistical comparisons of differences between the wild-type(WT), OsERF93 overexpression lines, and knockout lines for traits including plant height (C), tiller number (D), flag leaf length (E), flag leaf width (F), panicle length (G), primary branch number (H), secondary branch number (I), grain number per panicle (J), and 1000-grain weight (K). ** and * indicate statistically significant differences at P< 0.01 and P< 0.05, respectively.
| [1] | Kumar K V K, Reddy M S, Kloepper J W, Lawrence K S, Groth D E, Miller M E. Sheath blight disease of rice (Oryza sativa L.): An overview[J]. Biosciences Biotechnology Research Asia, 2016, 6(2): 465-480. |
| [2] | Nasimi Z, Barriuso J, Keshavarz T, Zheng A. Molecular, physiological, and biochemical properties of Sclerotia metamorphosis in Rhizoctonia solani[J]. Fungal Biology Reviews, 2024, 48: 100351. |
| [3] | 王爱军, 王娜, 顾思思, 赵文娟, 李平, 郑爱萍. 我国水稻纹枯病菌的融合类群及致病性差异[J]. 草业学报, 2018, 27 (7): 55-63. |
| Wang A J, Wang N, Gu S S, Zhao W J, Li P, Zheng A P. The fusion groups and pathogenicity differences of rice sheath blight pathogen in China[J]. Acta Prataculturae Sinica, 2018, 27(7): 55-63. (in Chinese with English abstract) | |
| [4] | Wu W, Huang J, Cui K, Nie L, Wang Q, Yang F, Shah F, Yao F, Peng S. Sheath blight reduces stem breaking resistance and increases lodging susceptibility of rice plants[J]. Field Crops Research, 2012, 128: 101-108. |
| [5] | 姜伟. 水稻纹枯病对产量和抗倒性的影响研究与抗病分子改良[D]. 扬州: 扬州大学, 2015. |
| Jiang W. Study on the effects of rice sheath blight on yield and lodging resistance, and molecular breeding for disease resistance[D]. Yangzhou: Yangzhou University, 2015. (in Chinese with English abstract) | |
| [6] | 陈海霞, 朱明芬, 常晓丽. 4种杀菌剂防治水稻纹枯病效果研究[J]. 上海农业学报, 2019, 35(2): 52-55. |
| Chen H X, Zhu M F, Chang X L. Study on the effect of four fungicides on sheath blight of rice[J]. Acta Agriculturae Shanghai, 2019, 35(2): 52-55. (in Chinese with English abstract) | |
| [7] | 袁筱萍, 魏兴华, 余汉勇, 王一平, 汤圣祥. 不同品种及有关外因对水稻纹枯病抗性的影响[J]. 作物学报, 2004, 30(8): 768-773. |
| Yuan X P, Wei X H, Yu H Y, Wang Y P, Tang S X. Influence of different varieties and related external factors on the resistance of rice to sheath blight[J]. Acta Agronomica Sinica, 2004, 30(8): 768-773. (in Chinese with English abstract) | |
| [8] | 桑海旭, 王井士, 刘郁, 李运动, 马晓慧, 刘志恒. 水稻纹枯病对水稻产量及米质的影响[J]. 北方水稻, 2013(1): 10-13. |
| Sang H X, Wang J S, Liu Y, Li Y D, Ma X H, Liu Z H. The impact of rice sheath blight on rice yield and rice quality[J]. Northern Rice, 2013(1): 10-13. (in Chinese with English abstract) | |
| [9] | 秦息林. 水稻纹枯病对水稻产量的影响[J]. 南方农业, 2015, 9(3): 27-28. |
| Qin X L. The impact of rice sheath blight on rice yield[J]. Southern Agriculture, 2015, 9(3): 27-28. (in Chinese) | |
| [10] | Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice[J]. Plant Physiology, 2006, 140(2): 411-432. |
| [11] | Xie W, Ding C, Hu H, Dong G, Zhang G, Qian Q, Ren D. Molecular events of rice AP2/ERF transcription factors[J]. International Journal of Molecular Sciences, 2022, 23(19): 12013. |
| [12] | Shigyo M, Ito M. Analysis of gymnosperm two-AP2-domain-containing genes[J]. Development Genes and Evolution, 2004, 214(3): 105-114. |
| [13] | Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress[J]. The Plant Cell, 1994, 6(2): 251-264. |
| [14] | Agarwal P K, Agarwal P, Reddy M K, Sopory S K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants[J]. Plant Cell Reports, 2006, 25(12): 1263-1274. |
| [15] | Tezuka D, Kawamata A, Kato H, Saburi W, Mori H, Imai R. The rice ethylene response factor OsERF83 positively regulates disease resistance to Magnaporthe oryzae[J]. Plant Physiology and Biochemistry, 2019, 135: 263-271. |
| [16] | Feng K, Hou X L, Xing G M, Liu J X, Duan A Q, Xu Z S, Li M Y, Zhuang J, Xiong A S. Advances in AP2/ERF super-family transcription factors in plant[J]. Critical Reviews in Biotechnology, 2020, 40(6): 750-776. |
| [17] | Kitomi Y, Hidemi K, Inukai Y. Molecular mechanism of crown root initiation and the different mechanisms between crown root and radicle in rice[J]. Plant Signaling & Behavior, 2011, 6(9): 1276-1278. |
| [18] | Lee D Y, An G. Two ap2 family genes, supernumerary bract (snb) and OsINDETERMINATE spikelet 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice[J]. The Plant Journal, 2012, 69(3): 445-461. |
| [19] | Jung H, Chung P J, Park S H, Redillas M C F R, Kim Y S, Suh J W, Kim J K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance[J]. Plant Biotechnology Journal, 2017, 15(10): 1295-1308. |
| [20] | Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou D X. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling[J]. The Plant Cell, 2015, 27(9): 2469-2483. |
| [21] | Lee D Y, Lee J, Moon S, Park S Y, An G. The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem[J]. The Plant Journal, 2007, 49(1): 64-78. |
| [22] | Guo H, Ecker J R. The ethylene signaling pathway: New insights[J]. Current Opinion in Plant Biology, 2004, 7(1): 40-49. |
| [23] | Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses[J]. Annual Review of Plant Biology, 2006, 57: 781-803. |
| [24] | Tran T T, Pérez-Quintero A L, Wonni I, Carpenter S C D, Yu Y, Wang L, Leach J E, Verdier V, Cunnac S, Bogdanove A J, Koebnik R, Hutin M, Szurek B. Functional analysis of African Xanthomonas oryzae pv. oryzae TALomes reveals a new susceptibility gene in bacterial leaf blight of rice[J]. PLoS Pathogens, 2018, 14(6): e1007092. |
| [25] | Takeuchi K, Gyohda A, Tominaga M, Kawakatsu M, Hatakeyama A, Ishii N, Shimaya K, Nishimura T, Riemann M, Nick P, Hashimoto M, Komano T, Endo A, Okamoto T, Jikumaru Y, Kamiya Y, Terakawa T, Koshiba T. RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots[J]. Plant & Cell Physiology, 2011, 52(9): 1686-1696. |
| [26] | Xie W, Cao W, Lu S, Zhao J, Shi X, Yue X, Wang G, Feng Z, Hu K, Chen Z, Zuo S. Knockout of transcription factor OsERF65 enhances ROS scavenging ability and confers resistance to rice sheath blight[J]. Molecular Plant Pathology, 2023, 24(12): 1535-1551. |
| [27] | Zarbafi S S, Ham J H. An overview of rice QTLs associated with disease resistance to three major rice diseases: Blast, sheath blight, and bacterial panicle blight[J]. Agronomy, 2019, 9(4): 177. |
| [28] | Channamallikarjuna V, Sonah H, Prasad M, Rao G J N, Chand S, Upreti H C, Singh N K, Sharma T R. Identification of major quantitative trait loci qSBR11-1 for sheath blight resistance in rice[J]. Molecular Breeding, 2010, 25(1): 155-166. |
| [29] | Helliwell E E, Wang Q, Yang Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia Solani[J]. Plant Biotechnology Journal, 2013, 11(1): 33-42. |
| [30] | Tonnessen B W, Manosalva P, Lang J M, Baraoidan M, Bordeos A, Mauleon R, Oard J, Hulbert S, Leung H, Leach J E. Rice phenylalanine ammonia-lyase gene OsPAL4 is associated with broad spectrum disease resistance[J]. Plant Molecular Biology, 2015, 87(3): 273-286. |
| [31] | Liu Y, Liu W W, Li L, Frederic F, Wang X F. Transcriptome analysis reveals different response of resistant and susceptible rice varieties to rice stripe virus infection[J]. Journal of Integrative Agriculture, 2023, 22(6): 1750-1762. |
| [32] | Yang X, Yan S, Li Y, Li G, Sun S, Li J, Cui Z, Huo J, Sun Y, Wang X, Liu F. Comparison of transcriptome between tolerant and susceptible rice cultivar reveals positive and negative regulators of response to Rhizoctonia Solani in rice[J]. International Journal of Molecular Sciences, 2023, 24(18): 14310. |
| [33] | Xue X, Cao Z X, Zhang X T, Wang Y, Zhang Y F, Chen Z X, Pan X B, Zuo S M. Overexpression of OsOSM1 enhances resistance to rice sheath blight[J]. Plant Disease, 2016, 100(8): 1634-1642. |
| [34] | Cao W, Cao X, Zhao J, Zhang Z, Feng Z, Ouyang S, Zuo S. Comprehensive characteristics of microRNA expression profile conferring to sheath blight pathogen Rhizoctonia solani in rice[J]. Rice Science, 2020, 27(2): 101-112. |
| [35] | Xie Y, Xie W, Zhao J, Xue X, Cao W, Shi X, Wang Z, Wang Y, Wang G, Feng Z, Hu K, Chen X, Chen Z, Zuo S. OsERF7 negatively regulates resistance to sheath blight disease by inhibiting phytoalexin biosynthesis[J]. Rice Science, 2025, 32(3): 367-379. |
| [36] | 贺闽, 尹俊杰, 冯志明, 朱孝波, 赵剑华, 左示敏, 陈学伟. 水稻稻瘟病和纹枯病抗性鉴定方法[J]. 植物学报, 2020, 55(5): 577-587. |
| He M, Yin J J, Feng Z M, Zhu X B, Zhao J H, Zuo S M, Chen X W. Identification methods for resistance to rice blast and sheath blight in rice[J]. Journal of Botany, 2020, 55(5): 577-587. (in Chinese with English abstract) | |
| [37] | 王志鹏. 水稻纹枯病的发生与防控技术[J]. 种子科技, 2022, 40 (6): 118-120. |
| Wang Z P. Occurrence and control techniques of rice sheath blight[J]. Seed Science and Technology, 2022, 40(6): 118-120. (in Chinese) | |
| [38] | 吴婕, 席亚东, 李洪浩, 王晓黎, 彭化贤. 四川省水稻纹枯病菌对井冈霉素抗药性监测[J]. 西南农业学报, 2015, 28 (6): 2501-2504. |
| Wu J, Xi Y D, Li H H, Wang X L, Peng H X. Monitoring of resistance to Jinggangmycin in Rhizoctonia solani causing rice sheath blight in Sichuan Province[J]. Journal of Southwest Agriculture, 2015, 28(6): 2501-2504. (in Chinese with English abstract) | |
| [39] | 吴志明, 李昆太. 水稻纹枯病的危害及其微生物防治概述[J]. 生物灾害科学, 2018, 41 (2): 81-88. |
| Wu Z M, Li K T. Overview of the harm of rice sheath blight and its microbiological control[J]. Journal of Biological Disaster Science, 2018, 41(2): 81-88. (in Chinese with English abstract) | |
| [40] | Huang Y, Li W, Liu T, Lin X, Xia Y, Zhu W, Cai Q. Rice extracellular vesicles send defense proteins into fungus Rhizoctonia solani to reduce disease[J]. Developmental Cell, 2025, 60(8): 1168-1181. |
| [41] | Cao B, Wang J, Ma J, Hai Y, Wang X, Fu Z, Xiang Z, Wang Y, Zhang L, Wang J, Li S. Large-scale screening and function analysis of Rhizoctonia Solani effectors targeting rice chloroplasts[J]. Journal of Agricultural and Food Chemistry, 2024, 72(44): 24336-24346. |
| [42] | Gutterson N, Reuber T L. Regulation of disease resistance pathways by AP2/ERF transcription factors[J]. Current Opinion in Plant Biology, 2004, 7(4): 465-471. |
| [43] | 许世达, 耿兴敏, 王露露. 植物乙烯响应因子(ERF)的结构、功能及表达调控研究进展[J]. 浙江农林大学学报, 2021, 38(3): 624-633. |
| Xu S D, Geng X M, Wang L L. Research progress on the structure, function, and expression regulation of plant ethylene response factors (ERF)[J]. Journal of Zhejiang A&F University, 2021, 38(3): 624-633. (in Chinese with English abstract) | |
| [44] | Wang L, Liu W, Wang Y. Heterologous expression of Chinese wild grapevine VqERFs in Arabidopsis thaliana enhance resistance to Pseudomonas syringae pv. tomato DC3000 and to Botrytis cinerea[J]. Plant Science, 2020, 293: 110421. |
| [45] | Hughes P W. OsGSK2 integrates jasmonic acid and brassinosteroid signaling in rice[J]. The Plant Cell, 2020, 32(9): 2669-2670. |
| [46] | Liu D, Chen X, Liu J, Ye J, Guo Z. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance[J]. Journal of Experimental Botany, 2012, 63(10): 3899-3911. |
| [47] | Hong Y, Wang H, Gao Y, Bi Y, Xiong X, Yan Y, Wang J, Li D, Song F. ERF transcription factor OsBIERF3 positively contributes to immunity against fungal and bacterial diseases but negatively regulates cold tolerance in rice[J]. International Journal of Molecular Sciences, 2022, 23(2): 606. |
| [1] | 李兴沂, 陈玲, 邵建韬, 肖素勤, 李金璐, 付惠仙, 殷富有, 张建红, 程在全, 刘丽. 水稻产量与淀粉品质协同调控的分子遗传研究进展[J]. 中国水稻科学, 2026, 40(1): 1-17. |
| [2] | 王轶欣, 林参, 马刘洋, 陈龙, 奉保华, 倪深, 魏祥进, 贺记外, 陈天晓. 谷丙转氨酶基因OsAlaAT4调控水稻氮素吸收和产量[J]. 中国水稻科学, 2026, 40(1): 51-60. |
| [3] | 黄奇娜, 姜鸿瑞, 杨婕, 于坤宇, 杨长登, 梁燕. 种子休眠基因Sdr4的生物信息学分析与分子标记开发和应用[J]. 中国水稻科学, 2026, 40(1): 61-71. |
| [4] | 程朝平, 何旎清, 白康呈, 林少俊, 黄凤凰, 刘军化, 程祖锌, 黄成志, 杨德卫. 聚合稻瘟病抗性基因Pigm-1和Pid2的水稻三系不育系福梦A的选育与利用[J]. 中国水稻科学, 2026, 40(1): 72-84. |
| [5] | 刘亚萍, 董译词, 郑君妍, 邱绚, 刘鹏程, 叶亚峰, 刘斌美, 陈析丰, 马伯军. 水稻类病变早衰突变体lmes7的鉴定与基因精细定位[J]. 中国水稻科学, 2026, 40(1): 85-94. |
| [6] | 谢世民, 周誉株, 薛晓迪, 朱广飞, 孙良, 陈建能. 水稻钵苗取栽协同作业式移栽机构设计与试验[J]. 中国水稻科学, 2026, 40(1): 131-144. |
| [7] | 王娟, 吴丽娟, 洪海波, 姚志文, 王磊, 鄂志国. 水稻泛素结合酶E2的生物学功能研究进展[J]. 中国水稻科学, 2025, 39(6): 744-750. |
| [8] | 陶士博, 许娜, 徐正进, 刘畅, 徐铨. 水稻发芽期耐冷基因Cold6的克隆[J]. 中国水稻科学, 2025, 39(6): 751-759. |
| [9] | 陈伟, 叶元妹, 赵剑华, 冯志明, 陈宗祥, 胡珂鸣, 左示敏. 利用CRISPR/Cas9技术改良南粳46抽穗期[J]. 中国水稻科学, 2025, 39(6): 760-770. |
| [10] | 侯桂花, 周立国, 雷建国, 陈虹, 聂元元. 水稻OsRDR5基因功能及作用机制初步解析[J]. 中国水稻科学, 2025, 39(6): 779-788. |
| [11] | 陆帅, 陶涛, 刘冉, 周文玉, 曹蕾, 杨青青, 张明秋, 任鑫哲, 杨芝笛, 徐福祥, 环海东, 龚远航, 张皓程, 金素奎, 蔡秀玲, 高继平, 冷语佳. 水稻长护颖小粒突变体lsg8的表型鉴定与基因克隆[J]. 中国水稻科学, 2025, 39(6): 813-824. |
| [12] | 邓欢, 刘亚培, 王春连, 郭威, 陈析丰, 纪志远. 水稻抗白叶枯病新基因Xa49(t)的定位分析[J]. 中国水稻科学, 2025, 39(6): 825-831. |
| [13] | 郝雯倩, 蔡兴菁, 杨海东, 吴宇阳, 滕轩, 薛超, 龚志云. 不同类型组蛋白修饰在水稻响应非生物胁迫中的研究进展[J]. 中国水稻科学, 2025, 39(5): 575-585. |
| [14] | 王镜博, 苏畅, 冯晶, 姜思旭, 徐海, 崔志波, 赵明辉. 水稻OsAlR1基因耐铝性功能研究[J]. 中国水稻科学, 2025, 39(5): 615-623. |
| [15] | 韶也, 胡远艺, 彭彦, 毛毕刚, 刘慧敏, 唐婵娟, 雷斌, 唐丽, 余丽霞, 李文建, 罗武中, 罗治斌, 袁远涛, 李曜魁, 张丹, 周利斌, 柏连阳, 唐文帮, 赵炳然. 基于M1TDS靶向筛选技术的重离子束诱变定向改良杂交水稻卓两优1126性状的研究[J]. 中国水稻科学, 2025, 39(5): 624-634. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||