
Chinese Journal OF Rice Science ›› 2021, Vol. 35 ›› Issue (5): 427-438.DOI: 10.16819/j.1001-7216.2021.210303
• Research Papers • Previous Articles Next Articles
Xiaojie CHU1, Tao LU1, Hanfei YE1, Sheng WANG1, Han LIN1, Xianmei WU2, Rui HE2, Gang YAN1, Yuexing WANG2, Sanfeng LI2, Mei LU1, Haitao HU1,*( ), Yaolong YANG2,*(
), Yaolong YANG2,*( ), Yuchun RAO1,*(
), Yuchun RAO1,*( )
)
Received:2021-03-04
Revised:2021-04-12
Online:2021-09-10
Published:2021-09-10
Contact:
Haitao HU, Yaolong YANG, Yuchun RAO
褚晓洁1, 芦涛1, 叶涵斐1, 王盛1, 林晗1, 吴先美2, 何瑞2, 严钢1, 王跃星2, 李三峰2, 路梅1, 胡海涛1,*( ), 杨窑龙2,*(
), 杨窑龙2,*( ), 饶玉春1,*(
), 饶玉春1,*( )
)
通讯作者:
胡海涛,杨窑龙,饶玉春
基金资助:Xiaojie CHU, Tao LU, Hanfei YE, Sheng WANG, Han LIN, Xianmei WU, Rui HE, Gang YAN, Yuexing WANG, Sanfeng LI, Mei LU, Haitao HU, Yaolong YANG, Yuchun RAO. Cloning and Functional Analysis of Leaf Senescence Gene LPS1 in Oryza sativa[J]. Chinese Journal OF Rice Science, 2021, 35(5): 427-438.
褚晓洁, 芦涛, 叶涵斐, 王盛, 林晗, 吴先美, 何瑞, 严钢, 王跃星, 李三峰, 路梅, 胡海涛, 杨窑龙, 饶玉春. 水稻叶片衰老基因LPS1的克隆与功能研究[J]. 中国水稻科学, 2021, 35(5): 427-438.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2021.210303
| 引物名称 Primer name | 正向引物 Forward primer(5′-3′) | 反向引物 Reverse primer(5′-3′) | 用途 Use |
|---|---|---|---|
| M1 | CACACATTCTAAATTTGGAAAAAGG | TACGTGGCTACTTGGCGTTC | 精细定位Fine mapping |
| M2 | GCTGAAGAGCCTCCTCGAA | GGTATCATGAGAGCGAGTCTGA | 精细定位Fine mapping |
| M3 | ACCCATGACCATGAGACGAT | GGGTACTAGCCGTGCTTATCC | 精细定位Fine mapping |
| M4 | AGCCATTAGGGGCTTAGGAA | CCCCTGAGTGATATGCTTGG | 精细定位Fine mapping |
| M5 | TAAATTACATCGGCCGGAGA | CCCACCAAAGAAATCTCCAA | 精细定位Fine mapping |
| M6 | ATTTGGGGGAAAGTTTGCTT | AATAGTATGCGTGCGCTGTG | 精细定位Fine mapping |
| LPS1 | TCCTAAGAAGGGCTCGGAAA | CTGCCTCAACCACACTTACA | 载体构建Vector construction |
| LPS1-GFP | GCCCAGATCAACTAGTATGGAT CTATATGCAATTGAC | TCGAGACGTCTCTAGACGGGCTGC AGGGGATGCCGG | 载体构建Vector construction |
| Q-YGL1 | AACCTTACCGTCCTATTCCTT | CCATACATCTAACAGAGCACC | qRT-PCR |
| Q-CAO1 | GATCCATACCCGATCGACAT | CGAGAGACATCCGGTAGAGC | qRT-PCR |
| Q-NYC3 | TCTATCTAGGTGCCAAAGGC | ATTCTGGCACCTGCTGTTTC | qRT-PCR |
| Q-DVR | CGAGCCCAGGTTCATCAAGGTGC | CCTCCCGATCTTGCCGAACTC | qRT-PCR |
| Q-RLS1 | CTTGGGCTGTTGATGCAGC | CTTCAACACCCGCCTCGC | qRT-PCR |
| Q-OsABA1 | GGATGCCATTGAGTTTGGTT | TGGCTGACTGAAGTCTCTCG | qRT-PCR |
| Q-OsABA2 | AGCAAACCTGAAAGGTGTGGA | AAAGCCACCATCCACCATGA | qRT-PCR |
| Q-OsABA3 | GGGCAAGATTTTGTTCGGCA | AAGGGTACACTTGTTGCCCC | qRT-PCR |
| Q-OsNCED1 | ACCATGAAGTCCATGAGGCT | TCTCGTAGTCTTGGTCTTGG | qRT-PCR |
| Q-OsNCED2 | ATGGAAACGAGGATAGTGGT | CTTATTGTTGTGCGAGAAGT | qRT-PCR |
| Q-OsNCED3 | CTCCCAAACCATCCAAACCG | TGAGCATATCCTGGCGTCGT | qRT-PCR |
| Q-OsNCED5 | TCCGAGCTCCTCGTCGTGAA | AGGTGTTTTGGAATGAACCA | qRT-PCR |
| Q-OsZEP | GGATGCCATTGAGTTTGGTT | TGGCTGACTGAAGTCTCTCG | qRT-PCR |
| Q-OsZDS | CACGTGTTCTTCGGGTGTTA | ATGTAACGGAGCTCCCACAG | qRT-PCR |
| Q-OsABA80x1 | AAGCTGGCAAAACCAACATC | CCGTGCTAATACGGAATCCA | qRT-PCR |
| Q-OsABA80x2 | CTACTGCTGATGGTGGCTGA | CCCATGGCCTTTGCTTTAT | qRT-PCR |
| Q-OsABA80x3 | AGTACAGCCCATTCCCTGTG | ACGCCTAATCAAACCATTGC | qRT-PCR |
| Actin | CAGGCCGTCCTCTCTCTGTA | AAGGATAGCATGGGGGAGAG | qRT-PCR |
Table 1 Primers and sequences used in this study.
| 引物名称 Primer name | 正向引物 Forward primer(5′-3′) | 反向引物 Reverse primer(5′-3′) | 用途 Use |
|---|---|---|---|
| M1 | CACACATTCTAAATTTGGAAAAAGG | TACGTGGCTACTTGGCGTTC | 精细定位Fine mapping |
| M2 | GCTGAAGAGCCTCCTCGAA | GGTATCATGAGAGCGAGTCTGA | 精细定位Fine mapping |
| M3 | ACCCATGACCATGAGACGAT | GGGTACTAGCCGTGCTTATCC | 精细定位Fine mapping |
| M4 | AGCCATTAGGGGCTTAGGAA | CCCCTGAGTGATATGCTTGG | 精细定位Fine mapping |
| M5 | TAAATTACATCGGCCGGAGA | CCCACCAAAGAAATCTCCAA | 精细定位Fine mapping |
| M6 | ATTTGGGGGAAAGTTTGCTT | AATAGTATGCGTGCGCTGTG | 精细定位Fine mapping |
| LPS1 | TCCTAAGAAGGGCTCGGAAA | CTGCCTCAACCACACTTACA | 载体构建Vector construction |
| LPS1-GFP | GCCCAGATCAACTAGTATGGAT CTATATGCAATTGAC | TCGAGACGTCTCTAGACGGGCTGC AGGGGATGCCGG | 载体构建Vector construction |
| Q-YGL1 | AACCTTACCGTCCTATTCCTT | CCATACATCTAACAGAGCACC | qRT-PCR |
| Q-CAO1 | GATCCATACCCGATCGACAT | CGAGAGACATCCGGTAGAGC | qRT-PCR |
| Q-NYC3 | TCTATCTAGGTGCCAAAGGC | ATTCTGGCACCTGCTGTTTC | qRT-PCR |
| Q-DVR | CGAGCCCAGGTTCATCAAGGTGC | CCTCCCGATCTTGCCGAACTC | qRT-PCR |
| Q-RLS1 | CTTGGGCTGTTGATGCAGC | CTTCAACACCCGCCTCGC | qRT-PCR |
| Q-OsABA1 | GGATGCCATTGAGTTTGGTT | TGGCTGACTGAAGTCTCTCG | qRT-PCR |
| Q-OsABA2 | AGCAAACCTGAAAGGTGTGGA | AAAGCCACCATCCACCATGA | qRT-PCR |
| Q-OsABA3 | GGGCAAGATTTTGTTCGGCA | AAGGGTACACTTGTTGCCCC | qRT-PCR |
| Q-OsNCED1 | ACCATGAAGTCCATGAGGCT | TCTCGTAGTCTTGGTCTTGG | qRT-PCR |
| Q-OsNCED2 | ATGGAAACGAGGATAGTGGT | CTTATTGTTGTGCGAGAAGT | qRT-PCR |
| Q-OsNCED3 | CTCCCAAACCATCCAAACCG | TGAGCATATCCTGGCGTCGT | qRT-PCR |
| Q-OsNCED5 | TCCGAGCTCCTCGTCGTGAA | AGGTGTTTTGGAATGAACCA | qRT-PCR |
| Q-OsZEP | GGATGCCATTGAGTTTGGTT | TGGCTGACTGAAGTCTCTCG | qRT-PCR |
| Q-OsZDS | CACGTGTTCTTCGGGTGTTA | ATGTAACGGAGCTCCCACAG | qRT-PCR |
| Q-OsABA80x1 | AAGCTGGCAAAACCAACATC | CCGTGCTAATACGGAATCCA | qRT-PCR |
| Q-OsABA80x2 | CTACTGCTGATGGTGGCTGA | CCCATGGCCTTTGCTTTAT | qRT-PCR |
| Q-OsABA80x3 | AGTACAGCCCATTCCCTGTG | ACGCCTAATCAAACCATTGC | qRT-PCR |
| Actin | CAGGCCGTCCTCTCTCTGTA | AAGGATAGCATGGGGGAGAG | qRT-PCR |
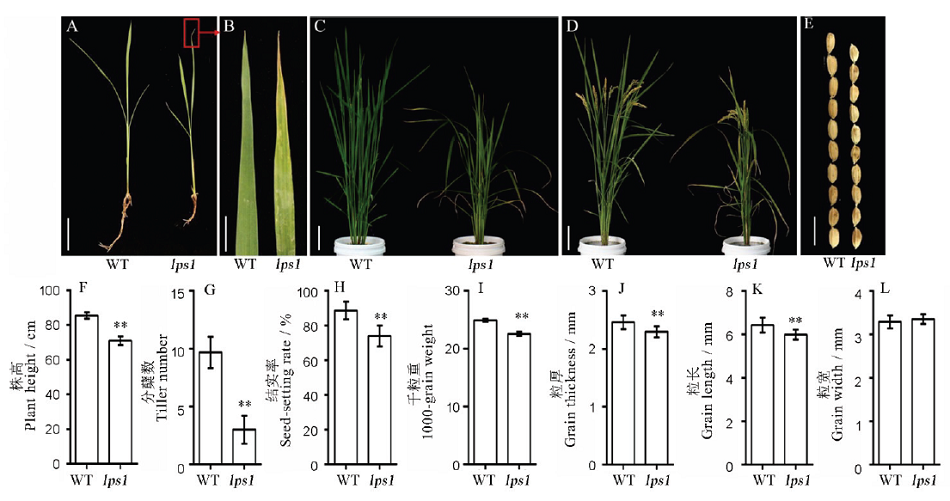
Fig. 1. Phenotype and agronomic traits of the wild type and lps1. A, Plant phenotype(Bar=3 cm); B, Leaf phenotype at the three-leaf stage(Bar=1 cm); C, Tillering stage(Bar=6 cm); D, Maturity stage(Bar=8 cm); E, Seed phenotype of wild type and lps1(Bar=10 cm); F-I, Agronomic traits of wild type and lps1; J-L, Statistics of seed thickness, length and width of the wild type and lps1. * and ** indicate that the wild-type and mutant lps1 are significantly different at the levels of 0.05 and 0.01, respectively.
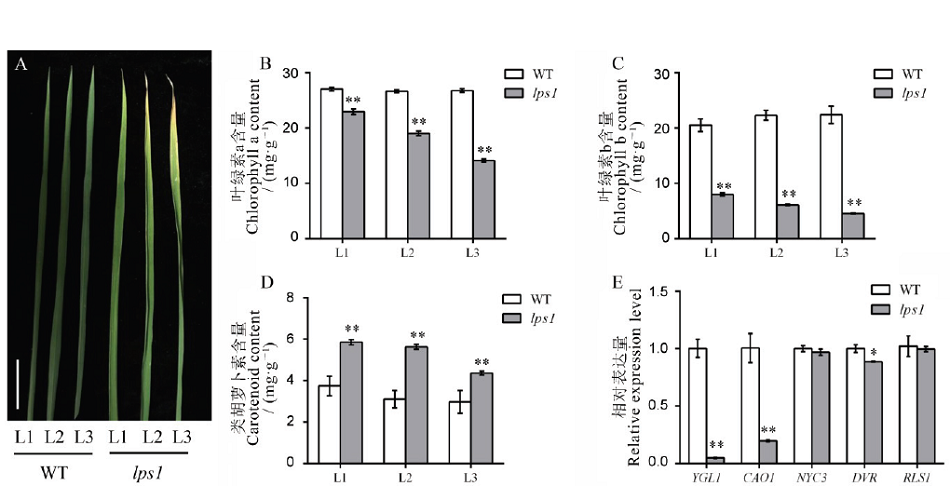
Fig. 2. Chlorophyll contents and related gene expression analysis at the tillering stage.A, Leaf phenotype of the wild type and lps1 in the tillering stage, bar=2 cm; B-D, Chlorophyll a, chlorophyll b and carotenoid contents of the wild type and lps1; E, Chlorophyll synthesis and metabolism-related gene expression level. L1, First leaf from the top; L2, Second leaf from the top; L3, Third leaf from the top. * and ** indicate that the wild type and lps1 are significantly different at the levels of 0.05 and 0.01, respectively.
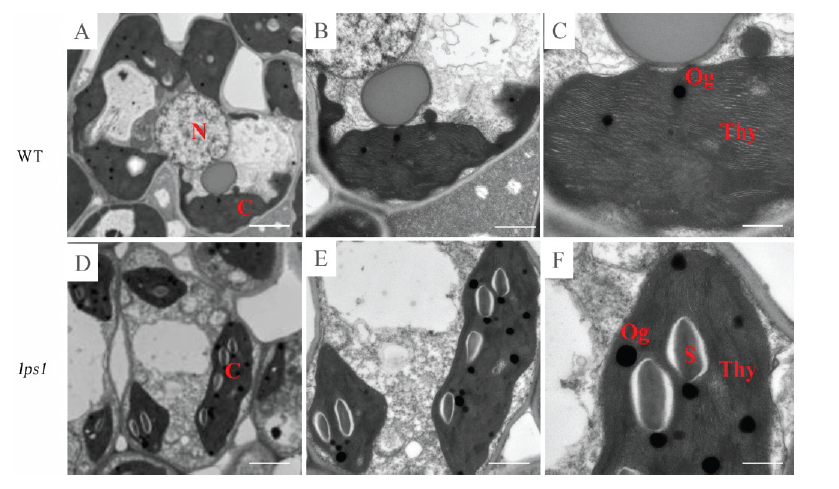
Fig. 4. Transmission electron microscopy (TEM) observation of the wild type (A-C) and lps1(D-F) leaves. N, Nucleus; C, Chloroplast; Thy, Thylakoid; S, Starch granules; Og, Osmophilic granules.
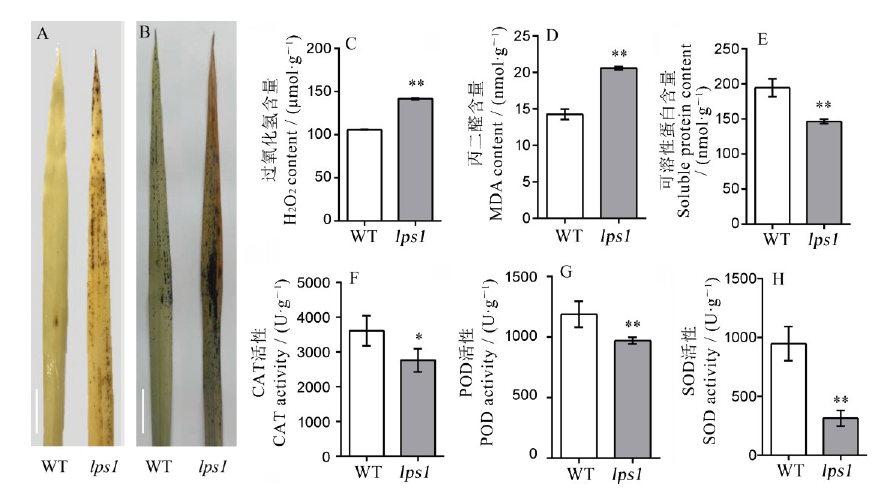
Fig. 5. Physiological and biochemical detection of the wild type and lps1.A, DAB staining; bar=2 cm; B, NBT staining, bar=2 cm; C, Determination of aging-related physiological indicators (H2O2, MDA, SP, CAT, POD, SOD). * and ** indicate that the wild-type and lps1 are significantly different at the levels of 0.05 and 0.01, respectively.
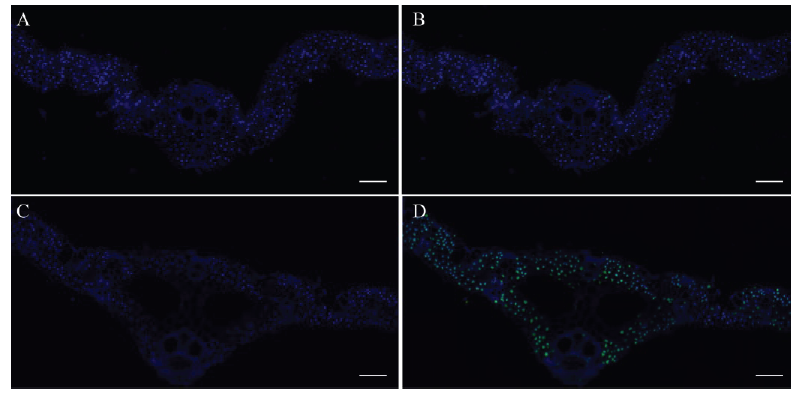
Fig. 6. TUNEL treatment results of the wild type (A and B) and lps1 (C and D) leaves. A-D, bar=100 μm. Blue fluorescence represents normal cells, green fluorescence represents apoptotic cells.
| 杂交组合 Combination | F1表型 Phenotype of F1 | F2表型 Phenotype of F2 | χ2(3:1) | ||
|---|---|---|---|---|---|
| 正常表型 Normal | 突变表型 Mutant | 总株数 Total | |||
| lps1/浙辐802 lps1/Zhefu 802 | 正常表型 Normal | 253 | 82 | 335 | 0.049 |
| 浙辐802/lps1 Zhefu 802/lps1 | 正常表型 Normal | 233 | 76 | 309 | 0.027 |
Table 2 Statistical results of F2 segregating population.
| 杂交组合 Combination | F1表型 Phenotype of F1 | F2表型 Phenotype of F2 | χ2(3:1) | ||
|---|---|---|---|---|---|
| 正常表型 Normal | 突变表型 Mutant | 总株数 Total | |||
| lps1/浙辐802 lps1/Zhefu 802 | 正常表型 Normal | 253 | 82 | 335 | 0.049 |
| 浙辐802/lps1 Zhefu 802/lps1 | 正常表型 Normal | 233 | 76 | 309 | 0.027 |
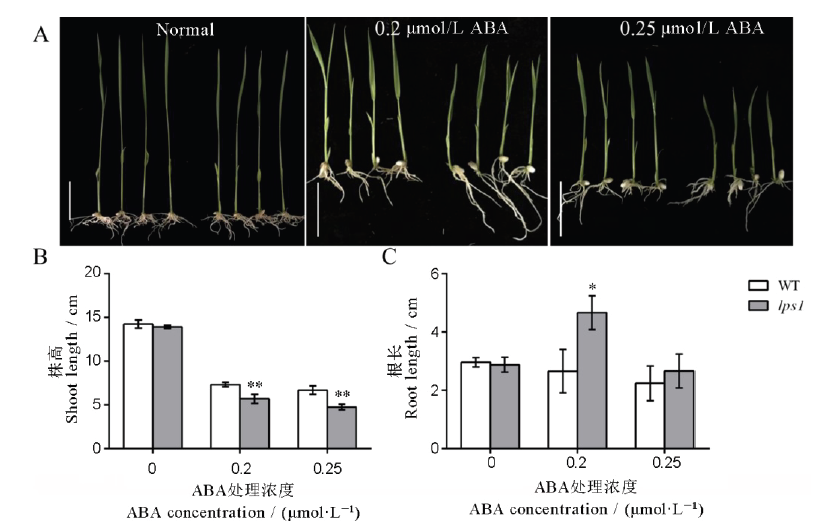
Fig. 9. Effect of exogenous hormone treatment on the wild type and lps1 seedlings. A, Effect of exogenous hormone treatment on the phenotype of the wild type(left) and lps1(right), bar=3 cm; B, Length of the aerial part after hormone treatment; C, Length of the underground part after hormone treatment. * and ** indicate significant difference between the wild type and lps1 at 0.05 and 0.01 level, respectively.
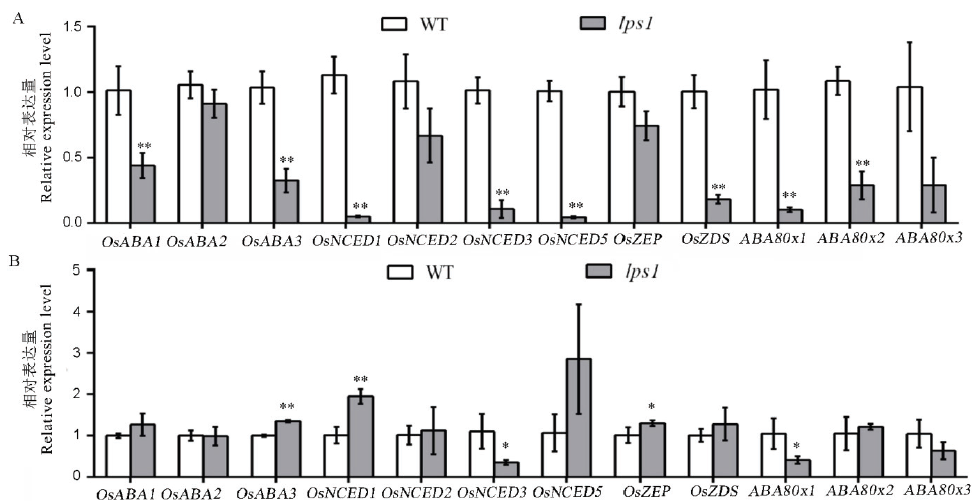
Fig. 10. ABA synthesis and degradation related gene expression levels. A, ABA-related gene expression levels in the wild type and lps1 before treatment; B, ABA-related gene expression levels in the wild type and lps1 after treatment; * and ** indicate significant difference between the wild type and lps1 at 0.05 and 0.01 level, respectively.
| [1] | Cao J, Jiang F, Sodmergen, Cui K M. Time-course of programmed cell death during leaf senescence in Eucommia ulmoides[J]. Journal of Plant Research, 2003, 116(1): 7-12. |
| [2] | Vicky B W. The molecular biology of leaf senescence[J]. Journal of Experimental Botany, 1997, 48(2): 181-199. |
| [3] | Lim P O, Kim H J, Nam H G. Leaf senescence[J]. Annual Review of Plant Biology, 2007, 58(1): 115-136. |
| [4] | Zhang H S, Zhou C J. Signal transduction in leaf senescence[J]. Plant Molecular Biology, 2013, 82(6): 539-545. |
| [5] | Panda D, Sarkar R K. Natural leaf senescence: Probed by chlorophyll fluorescence, CO2 photosynthetic rate and antioxidant enzyme activities during grain filling in different rice cultivars[J]. Physiology and Molecular Biology of Plants, 2013, 19(3): 43-51. |
| [6] | Li Z H, Zhang Y, Zou D, Zhao Y, Wang H L, Zhang Y, Xia X L, Luo J C, Zhang Z. LSD 3.0: A comprehensive resource for the leaf senescence research community[J]. Nucleic Acids Research, 2020, 48(D1): D1069-D1075. |
| [7] | Mao C J, Lu S C, Lü B, Zhang B, Shen J B, He J M, Luo L Q, Xi D D, Chen X, Ming F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis[J]. Plant Physiology, 2017, 174(3): 1747-1763. |
| [8] | Liang C Z, Wang Y Q, Zhu Y N, Tang J Y, Hu B, Liu L C, Ou S J, Sun X H, Chu J F, Chu C C. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(27): 10013-10018. |
| [9] | Han M, Kim C Y, Lee J, Lee S K, Jeon J S. OsWRKY42 represses OsMT1d and induces reactive oxygen species and leaf senescence in rice[J]. Molecular Cells, 2014, 37(7): 532-539. |
| [10] | Uji Y, Akimitsu K, Gomi K. Identification of OsMYC2-regulated senescence-associated genes in rice[J]. Planta, 2017, 245(6): 1241-1246. |
| [11] | Lu G W, Casaretto J A, Ying S, Mahmood K, Liu F, Bi Y M, Rothstein S J. Overexpression of OsGATA12 regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting[J]. Plant Molecular Biology, 2017, 94(1): 215-227. |
| [12] | Liu W Z, Fu Y P, Hu G C, Si H M, Wu C, Sun Z X. Identification and fine mapping of a thermo-sensitive chlorophyll deficient mutant in rice (Oryza sativa L.)[J]. Planta, 2007, 226(3): 785-795. |
| [13] | Liu C H, Zhu H T, Xing Y, Tan J J, Chen X H, Zhang J J, Peng H F, Xie Q J, Zhang Z M. Albino Leaf 2 is involved in the splicing of chloroplast group I and II introns in rice[J]. Journal of Experimental Botany, 2016, 67(18): 5339-5347. |
| [14] | Kusaba M, Ito H, Morita R, Iida S, Sato Y, Fujimoto M, Kawasaki S, Tanaka R, Hirochika H, Nishimura M, Tanaka A. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence[J]. Plant Cell, 2007, 19(4): 1362-1375. |
| [15] | Rong H, Tang Y Y, Zhang H, Wu P Z, Chen Y P, Li M R, Wu G J, Jiang H W. The Stay-Green Rice like (SGRL) gene regulates chlorophyll degradation in rice[J]. Journal of Plant Physiology, 2013, 170(15): 1367-1373. |
| [16] | Fang C Y, Zhang H, Wan J, Wu Y Y, Chen W, Wang S C, Wang W S, Zhang H W, Zhang F, Qu L H, Liu X Q, Zhou D X, Luo J. Control of leaf senescence by an MeOH-jasmonates cascade that is epigenetically regulated by OsSRT1 in rice[J]. Molecular Plant, 2016, 9(10): 1366-1378. |
| [17] | Xu W Y, Kong Z S, Li M N, Yang W Q, Xue Y B. A novel nuclear-localized CCCH-type ainc finger protein, OsDOS, is involved in delaying leaf senescence in rice[J]. Plant Physiology, 2006, 141(4): 1376-1388. |
| [18] | Chen Y, Xu Y Y, Luo W, Li W X, Chen N, Zhang D J, Chong K. The F-Box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence, in rice[J]. Plant Physiology, 2013, 163(4): 1673-1685. |
| [19] | Zhao Y, Chan Z L, Gao J H, Xing L, Cao M J, Yu C M, Hu Y L, You J, Shi H T, Zhu Y F, Gong Y H, Mu Z X, Wang H Q, Deng X, Wang P C, Bressan R A, Zhu J K. ABA receptor PYL9 promotes drought resistance and leaf senescence[J]. Proceedings of the National Academy of Sciences of The United States of America, 2016, 113(7): 1949-1954. |
| [20] | Yang X, Gong P, Li K Y, Huang F D, Cheng F M, Pan G. A single cytosine deletion in the OsPLS1 gene encoding vacuolar-type H+-ATPase subunit A1 leads to premature leaf senescence and seed dormancy in rice[J]. Journal of Experimental Botany, 2016, 67(9): 2761-2776. |
| [21] | Zhou Y, Liu L, Huang W F, Yuan M, Zhou F, Li X H, Lin Y J. Overexpression of OsSWEET5 in rice causes growth retardation and precocious senescence[J]. PloS One, 2014, 9(4): e94210. |
| [22] | Singh S, Singh A, Nandi A K. The rice OsSAG12-2 gene codes for a functional protease that negatively regulates stress-induced cell death[J]. Journal of Biosciences, 2016, 41(3): 445-453. |
| [23] | Wu L W, Ren D Y, Hu S K, Li G M, Dong G J, Jiang L, Hu X M, Ye W J, Cui Y T, Zhu J, Zhang G H, Gao Z Y, Zeng D L, Qian Q, Guo L B. Down-regulation of a nicotinate phosphoribosyltransferase gene OsNaPRT1 leads to withered leaf tips[J]. Plant Physiology, 2016, 171(2): 1085-1098. |
| [24] | Lee R H, Hsu J H, Huang H J, Lo S F, Grace S C. Alkaline α-galactosidase degrades thylakoid membranes in the chloroplast during leaf senescence in rice[J]. New Phytology, 2009, 184(3): 596-606. |
| [25] | Kang K, Kim Y S, Park S, Back K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves[J]. Plant Physiology, 2009, 150(3): 1380-1393. |
| [26] | Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin glucosyltransferase in rice[J]. Plant Physiology, 2012, 160(1): 319-331. |
| [27] | Wiseman B R. Plant-resistance to insects in integrated pest-management[J]. Plant Disease, 1994, 78(9): 927-932. |
| [28] | Liang C Z, Chu C C. Towards understanding abscisic acid-mediated leaf senescence[J]. Science China: Life Sciences, 2015, 58(5): 506-508. |
| [29] | Pan H R, Liu S M, Tang D Z. HPR1, a component of the THO/TREX complex, plays an important role in disease resistance and senescence in Arabidopsis[J]. Plant Journal, 2012, 69(5): 831-843. |
| [30] | Hu Y N, Jiang Y J, Han X, Wang H P, Pan J J, Yu D Q. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones[J]. Journal of Experimental Botany, 2017, 68(6): 1361-1369. |
| [31] | Dani K G S, Fineschi S, Michelozzi M, Loreto F. Do cytokinins, volatile isoprenoids and carotenoids synergically delay leaf senescence[J]. Plant Cell Environment, 2016, 39(5): 1103-1111. |
| [32] | Kim J I, Murphy A S, Baek D, Lee S W, Yun D J, Bressan R A, Narasimhan M L. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana[J]. Journal of Experimental Botany, 2011, 62(11): 3981-3992. |
| [33] | Chen L G, Xiang S Y, Chen Y L, Li D B, Yu D Q. Arabidopsis WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence[J]. Molecular Plant, 2017, 10, 1174-1189. |
| [34] | Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism[J]. Plant Cell, 2006, 18(1): 40-54. |
| [35] | Wang T, Li C X, Wu Z H, Jia Y C, Wang H, Sun S Y, Mao C Z, Wang X L. Abscisic acid regulates auxin homeostasis in rice root tips to promote root hair elongation[J]. Frontier in Plant Science, 2017, 8: 1121-1138. |
| [36] | Xu N, Chu Y L, Chen H L, Li X X, Wu Q, Jin L, Wang G X, Huang J L. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging[J]. PLoS Genet, 2018, 14(10): e1007662. |
| [37] | Chang Y, Nguyen B H, Xie Y J, Xiao B Z, Tang N, Zhu W L, Mou T M, Xiong L Z. Co-overexpression of the constitutively active form of OsbZIP46 and ABA-activated protein kinase SAPK6 improves drought and temperature stress resistance in rice[J]. Frontier in Plant Science, 2017, 8: 1102-1117. |
| [38] | Promchuea S, Zhu Y J, Chen Z Z, Zhang J, Gong Z Z. ARF2 coordinates with PLETHORAs and PINs to orchestrate ABA-mediated root meristem activity in Arabidopsis[J]. Journal of Integrative Plant Biology, 2017, 59(1): 30-43. |
| [39] | Sun L R, Wang Y B, He S B, Hao F S. Mechanisms for abscisic acid inhibition of primary root growth[J]. Plant Signaling & Behavior, 2018, 13(9): e1500069. |
| [40] | Hung K T, Yi T H, Kao C H. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves[J]. Physiology Plantarum, 2006, 127(2): 293-303. |
| [41] | 李兆伟. 水稻叶片早衰突变体的糖代谢基因表达与抗氧化生理调控[D]. 杭州: 浙江大学, 2014. |
| Li Z W. The expression alteration of various genes related to sugar metabolism in senescing leaves and its antioxidation modulation for esl mutant[D]. Hangzhou: Zhejiang University, 2014. | |
| [42] | Lichtenthaler H K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes[J]. Methods in Enzymology, 1987, 148: 350-382. |
| [43] | 游均, 方玉洁, 熊立仲. 活性氧检测[J/OL].Bio-101, 2018: e1010170. |
| You J, Fang Y J, Xiong L Z. Reactive oxygen detection [J/OL]. Bio-101, 2018: e1010170. (in Chinese) | |
| [44] | Rao Y C, Jiao R, Wang S, Wu X M, Ye H F, Pan C Y, Li S F, Xin D D, Zhou W Y, Dai G X, Hu J, Ren D Y, Wang Y X. SPL36 encodes a receptor-like protein kinase that regulates programmed cell death and defense responses in rice[J]. Rice, 2021, 14(1): 34-47. |
| [45] | 沈恒胜, 陈君琛, 黄进华, 汤葆莎. 水稻叶表皮硅体显微结构及其分布[J]. 福建农业大学学报, 2005, 34(2): 137-140. |
| Shen H S, Chen J C, Huang J H, Tang B S. Microtructure and distribution of silica bodies rice epidermis[J]. Fujian Academy of Agricultural Sciences, 2005, 34(2): 137-140. (in Chinese with English abstract) | |
| [46] | 杨秉耀, 陈新芳, 刘向东, 郭海滨. 水稻不同品种叶表面硅质细胞的扫描电镜观察[J]. 电子显微学报, 2006, 25(2): 68-72. |
| Yang B Y, Chen X F, Liu X D, Guo H B. Scanning electron microscopic observation of silicon cells on the leaf surface of different rice varieties[J]. Journal of Chinese Electron Microscopy Society, 2005, 25(2): 68-72. | |
| [47] | Christopher E. A possible mechanism of biological silicification in plants[J]. Frontier in Plant Science, 2015, 6: 853-859. |
| [48] | Yang L, Han Y Q, Li P, Li F, Ali S, Hou M L. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder[J]. Scientific Report, 2017, 7(1): 4232-4240. |
| [49] | Hideg E, Kálai T, Kós P B, Asada K, Hideg K. Singletoxygen in plants: Its significance and possible detection with double (fluorescent and spin) indicator reagents[J]. Photochemistry and Photobiology, 2006, 82(5): 1211-1218. |
| [50] | 华春, 王仁雷. 杂交稻及其三系叶片衰老过程中SOD、CAT活性和MDA含量的变化[J]. 西北植物学报, 2003, 23(3): 406-409. |
| Hua C, Wang R L. Changes of SOD and CAT activities and MDA content during senescence of hybrid rice and three lines leaves[J]. Acta Botanica Boreali- Occidentalia Sinica, 2003, 23(3): 406-409. | |
| [51] | Hu B, Zhu C G, Li F, Tang J Y, Wang Y Q, Liu L C, Che R H, Chu C C. LEAF TIP NECROSIS1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice[J]. Plant Physiology, 2011, 156(3): 1101-1115. |
| [52] | Lee I C, Hong S W, Whang S S, Lim P O, Nam H G, Koo J C. Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves[J]. Plant and Cell Physiology, 2011, 52(4): 651-662. |
| [1] | GUO Zhan, ZHANG Yunbo. Research Progress in Physiological,Biochemical Responses of Rice to Drought Stress and Its Molecular Regulation [J]. Chinese Journal OF Rice Science, 2024, 38(4): 335-349. |
| [2] | WEI Huanhe, MA Weiyi, ZUO Boyuan, WANG Lulu, ZHU Wang, GENG Xiaoyu, ZHANG Xiang, MENG Tianyao, CHEN Yinglong, GAO Pinglei, XU Ke, HUO Zhongyang, DAI Qigen. Research Progress in the Effect of Salinity, Drought, and Their Combined Stresses on Rice Yield and Quality Formation [J]. Chinese Journal OF Rice Science, 2024, 38(4): 350-363. |
| [3] | XU Danjie, LIN Qiaoxia, LI Zhengkang, ZHUANG Xiaoqian, LING Yu, LAI Meiling, CHEN Xiaoting, LU Guodong. OsOPR10 Positively Regulates Rice Blast and Bacterial Blight Resistance [J]. Chinese Journal OF Rice Science, 2024, 38(4): 364-374. |
| [4] | CHEN Mingliang, ZENG Xihua, SHEN Yumin, LUO Shiyou, HU Lanxiang, XIONG Wentao, XIONG Huanjin, WU Xiaoyan, XIAO Yeqing. Typing of Inter-subspecific Fertility Loci and Fertility Locus Pattern of indica-japonica Hybrid Rice [J]. Chinese Journal OF Rice Science, 2024, 38(4): 386-396. |
| [5] | DING Zhengquan, PAN Yueyun, SHI Yang, HUANG Haixiang. Comprehensive Evaluation and Comparative Analysis of Jiahe Series Long-Grain japonica Rice with High Eating Quality Based on Gene Chip Technology [J]. Chinese Journal OF Rice Science, 2024, 38(4): 397-408. |
| [6] | HOU Xiaoqin, WANG Ying, YU Bei, FU Weimeng, FENG Baohua, SHEN Yichao, XIE Hangjun, WANG Huanran, XU Yongqiang, WU Zhihai, WANG Jianjun, TAO Longxing, FU Guanfu. Mechanisms Behind the Role of Potassium Fulvic Acid in Enhancing Salt Tolerance in Rice Seedlings [J]. Chinese Journal OF Rice Science, 2024, 38(4): 409-421. |
| [7] | LÜ Zhou, YI Binghuai, CHEN Pingping, ZHOU Wenxin, TANG Wenbang, YI Zhenxie. Effects of Nitrogen Application Rate and Transplanting Density on Yield Formation of Small Seed Hybrid Rice [J]. Chinese Journal OF Rice Science, 2024, 38(4): 422-436. |
| [8] | HU Jijie, HU Zhihua, ZHANG Junhua, CAO Xiaochuang, JIN Qianyu, ZHANG Zhiyuan, ZHU Lianfeng. Effects of Rhizosphere Saturated Dissolved Oxygen on Photosynthetic and Growth Characteristics of Rice at Tillering Stage [J]. Chinese Journal OF Rice Science, 2024, 38(4): 437-446. |
| [9] | WU Yue, LIANG Chengwei, ZHAO Chenfei, SUN Jian, MA Dianrong. Occurrence of Weedy Rice Disaster and Ecotype Evolution in Direct-Seeded Rice Fields [J]. Chinese Journal OF Rice Science, 2024, 38(4): 447-455. |
| [10] | LIU Fuxiang, ZHEN Haoyang, PENG Huan, ZHENG Liuchun, PENG Deliang, WEN Yanhua. Investigation and Species Identification of Cyst Nematode Disease on Rice in Guangdong Province [J]. Chinese Journal OF Rice Science, 2024, 38(4): 456-461. |
| [11] | CHEN Haotian, QIN Yuan, ZHONG Xiaohan, LIN Chenyu, QIN Jinghang, YANG Jianchang, ZHANG Weiyang. Research Progress on the Relationship Between Rice Root, Soil Properties and Methane Emissions in Paddy Fields [J]. Chinese Journal OF Rice Science, 2024, 38(3): 233-245. |
| [12] | MIAO Jun, RAN Jinhui, XU Mengbin, BO Liubing, WANG Ping, LIANG Guohua, ZHOU Yong. Overexpression of RGG2, a Heterotrimeric G Protein γ Subunit-Encoding Gene, Improves Drought Tolerance in Rice [J]. Chinese Journal OF Rice Science, 2024, 38(3): 246-255. |
| [13] | YIN Xiaoxiao, ZHANG Zhihan, YAN Xiulian, LIAO Rong, YANG Sijia, Beenish HASSAN, GUO Daiming, FAN Jing, ZHAO Zhixue, WANG Wenming. Signal Peptide Validation and Expression Analysis of Multiple Effectors from Ustilaginoidea virens [J]. Chinese Journal OF Rice Science, 2024, 38(3): 256-265. |
| [14] | ZHU Yujing, GUI Jinxin, GONG Chengyun, LUO Xinyang, SHI Jubin, ZHANG Haiqing, HE Jiwai. QTL Mapping for Tiller Angle in Rice by Genome-wide Association Analysis [J]. Chinese Journal OF Rice Science, 2024, 38(3): 266-276. |
| [15] | WEI Qianqian, WANG Yulei, KONG Haimin, XU Qingshan, YAN Yulian, PAN Lin, CHI Chunxin, KONG Yali, TIAN Wenhao, ZHU Lianfeng, CAO Xiaochuang, ZHANG Junhua, ZHU Chunqun. Mechanism of Hydrogen Sulfide, a Signaling Molecule Involved in Reducing the Inhibitory Effect of Aluminum Toxicity on Rice Growth Together with Sulfur Fertilizer [J]. Chinese Journal OF Rice Science, 2024, 38(3): 290-302. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||