
中国水稻科学 ›› 2019, Vol. 33 ›› Issue (5): 457-466.DOI: 10.16819/j.1001-7216.2019.9036
陈燕华1,3, 王亚梁1,2,*( ), 朱德峰1, 石庆华2, 陈惠哲1, 向镜1, 张义凯1, 张玉屏1,*(
), 朱德峰1, 石庆华2, 陈惠哲1, 向镜1, 张义凯1, 张玉屏1,*( )
)
收稿日期:2019-03-29
修回日期:2019-04-15
出版日期:2019-09-10
发布日期:2019-09-10
通讯作者:
王亚梁,张玉屏
基金资助:
Yanhua CHEN1,3, Yaliang WANG1,2,*( ), Defeng ZHU1, Qinghua SHI2, Huizhe CHEN1, Jing XIANG1, Yikai ZHANG1, Yuping ZHANG1,*(
), Defeng ZHU1, Qinghua SHI2, Huizhe CHEN1, Jing XIANG1, Yikai ZHANG1, Yuping ZHANG1,*( )
)
Received:2019-03-29
Revised:2019-04-15
Online:2019-09-10
Published:2019-09-10
Contact:
Yaliang WANG, Yuping ZHANG
摘要:
【目的】 明确水稻穗分化期高温下喷施2,4-表油菜素内酯(2,4-epibrassinolide, EBR)对穗生长及颖花形成的影响,并探究其生理机制。【方法】 以热敏感型水稻IR36为材料,在幼穗分化期设置40℃高温和32℃适温两个处理,并喷施EBR,研究幼穗碳水化合物供应、蔗糖代谢、细胞分裂素代谢及抗氧化能力的变化。【结果】 1)高温和适温喷施EBR,水稻每穗粒数分别比不喷施的对照增加13.7% 和45.7%,其中以喷施0.15 mg/L效果最好,缓解了高温对水稻幼穗生长的抑制,增加颖花分化数和降低颖花退化率。2)喷施EBR对叶片净光合速率无显著影响,但促进幼穗中干物质和非结构性碳水化合物积累。EBR喷施增加高温下幼穗中蔗糖转运基因OsSUT1、OsSUT2和OsSUT4的表达,并显著提高蔗糖代谢相关酶活性,EBR对高温下碳水化合物利用的促进作用大于适温处理。3)喷施EBR降低高温下细胞分裂素氧化酶基因OsCKX5和OsCKX9的表达量,同时促进细胞分裂素合成和信号调节相关基因的表达,并在适温下也表现出类似的效应。4)喷施EBR降低高温下超氧阴离子含量,增强了超氧化物歧化酶、过氧化氢酶和过氧化物酶活性。【结论】 高温下,喷施适宜浓度的EBR促进碳水化合物向幼穗的转运,抑制细胞分裂素分解,同时降低高温引起的过氧化伤害,进而缓解了高温对颖花形成的伤害。适温条件喷施EBR也对颖花形成具有一定的促进作用。
中图分类号:
陈燕华, 王亚梁, 朱德峰, 石庆华, 陈惠哲, 向镜, 张义凯, 张玉屏. 外源油菜素内酯缓解水稻穗分化期高温伤害的机理研究[J]. 中国水稻科学, 2019, 33(5): 457-466.
Yanhua CHEN, Yaliang WANG, Defeng ZHU, Qinghua SHI, Huizhe CHEN, Jing XIANG, Yikai ZHANG, Yuping ZHANG. Mechanism of Exogenous Brassinolide in Alleviating High Temperature Injury at Panicle Initiation Stage in Rice[J]. Chinese Journal OF Rice Science, 2019, 33(5): 457-466.
| 基因Gene | 正向引物Forward primer 5' → 3' | 反向引物Reverse primer 5' → 3' |
|---|---|---|
| OsUBQ | AACCAGCTGAGGCCCAAGA | ACGATTGATTTAACCAGTCCATGA |
| OsSUT1 | CCACCTCGGTAGAAGAGAATAA | CCATTCATTACACACTAATTACCAA |
| OsSUT2 | AGGAGGAGAGGTCACCGATAA | CCAACATCCAATGTACAACAGCA |
| OsSUT4 | TTTGGCTGAGCAGAACACCA | ATGTCATTCGGGCAGAGCTT |
| OsLOGL2 | GAGCGCACAGAAAAGAGAAGC | GGCATGAGTGCTTTTGGAAT |
| OsLOGL3 | GTGCTGCATTGTCTGCAGTT | GGTCATGAGAGTCTTGGGGA |
| OsCKX5 | CGCTGCTGGGCGAGCTGAAT | CGCCTTGTGCACGCGGTCTA |
| OsCKX9 | GCCAGGATTCCTCTTGAACCTGC | ACGCACTGGGTCCTGCGGAT |
| OsRR2 | ACGATCTTCTCAAAGCCATCAAG | TGAGAGGCTTAAGGATGAAATCCT |
| OsRR5 | ACCGAATGTGAGCATGATTATCA | CCTTGACCTTCTTCAGGAGTTCATA |
| ORR2 | TGGGTAGTTCAAAGCTGCAG | GACTAGAAAAGGCGCTGACA |
| ORR4 | TCAGTGGTGGTCTAGATGAC | CGATGATTAACGAGAATTTTAC |
表1 实时荧光定量PCR引物序列
Table 1 Primers used for quantitative real-time PCR.
| 基因Gene | 正向引物Forward primer 5' → 3' | 反向引物Reverse primer 5' → 3' |
|---|---|---|
| OsUBQ | AACCAGCTGAGGCCCAAGA | ACGATTGATTTAACCAGTCCATGA |
| OsSUT1 | CCACCTCGGTAGAAGAGAATAA | CCATTCATTACACACTAATTACCAA |
| OsSUT2 | AGGAGGAGAGGTCACCGATAA | CCAACATCCAATGTACAACAGCA |
| OsSUT4 | TTTGGCTGAGCAGAACACCA | ATGTCATTCGGGCAGAGCTT |
| OsLOGL2 | GAGCGCACAGAAAAGAGAAGC | GGCATGAGTGCTTTTGGAAT |
| OsLOGL3 | GTGCTGCATTGTCTGCAGTT | GGTCATGAGAGTCTTGGGGA |
| OsCKX5 | CGCTGCTGGGCGAGCTGAAT | CGCCTTGTGCACGCGGTCTA |
| OsCKX9 | GCCAGGATTCCTCTTGAACCTGC | ACGCACTGGGTCCTGCGGAT |
| OsRR2 | ACGATCTTCTCAAAGCCATCAAG | TGAGAGGCTTAAGGATGAAATCCT |
| OsRR5 | ACCGAATGTGAGCATGATTATCA | CCTTGACCTTCTTCAGGAGTTCATA |
| ORR2 | TGGGTAGTTCAAAGCTGCAG | GACTAGAAAAGGCGCTGACA |
| ORR4 | TCAGTGGTGGTCTAGATGAC | CGATGATTAACGAGAATTTTAC |
| 处理 Treatment | EBR浓度 Concentration of EBR/(mg·L-1) | 单株穗数 Panicle number per plant | 每穗粒数 Number of spikelets per panicle | 结实率 Seed setting rate/% | 千粒重 1000-grain weight/g | 单株产量 Yield per plant /g |
|---|---|---|---|---|---|---|
| NT | 0.00 | 12.0±1.0 a | 100.7±11.2 a | 81.4±1.8 ab | 19.6±0.3 a | 19.1±0.1 b |
| 0.05 | 11.3±0.6 a | 105.5±4.4 b | 82.1±1.6 ab | 19.2±1.1 a | 18.8±1.3 b | |
| 0.10 | 12.3±0.6 a | 111.2±3.4 abc | 86.9±0.2 a | 19.4±1.1 a | 23.1±1.8 a | |
| 0.15 | 12.7±0.6 a | 121.0±5.2 a | 75.8±5.8 b | 18.6±0.8 a | 21.7±4.9 ab | |
| 0.30 | 12.3±1.5 a | 113.9±5.2 ab | 79.8±2.8 b | 19.1±0.8 a | 21.3±4.7 ab | |
| 0.50 | 13.3±1.5 a | 105.8±6.5 bc | 80.0±2.9 b | 19.1±0.7 a | 21.6±2.4 ab | |
| HT | 0.00 | 12.3±0.6 a | 52.5±0.9 d | 46.6±7.3 b | 18.0±1.0 a | 5.4±0.5 c |
| 0.05 | 13.0±2.0 a | 71.0±4.8 bc | 49.8±4.9 b | 17.6±0.3 a | 8.1±1.0 b | |
| 0.10 | 12.0±1.0 a | 83.2±3.1 a | 45.9±6.8 b | 17.7±0.2 a | 8.1±1.2 b | |
| 0.15 | 11.7±0.6 a | 85.9±4.2 a | 65.6±9.5 a | 17.7±0.3 a | 11.6±1.3 a | |
| 0.30 | 11.3±0.6 a | 76.5±1.6 b | 49.4±1.4 b | 18.1±0.5 a | 9.2±0.4 b | |
| 0.50 | 12.7±0.6 a | 65.7±4.7 c | 55.9±6.9 ab | 18.0±0.7 a | 8.4±1.6 b |
表2 高温下不同浓度EBR喷施对水稻单株产量及其构成的影响
Table 2 Rice yield and its components per plant under exposure to different concentrations of exogenous 2, 4-epibrassinolide (EBR) at high temperature.
| 处理 Treatment | EBR浓度 Concentration of EBR/(mg·L-1) | 单株穗数 Panicle number per plant | 每穗粒数 Number of spikelets per panicle | 结实率 Seed setting rate/% | 千粒重 1000-grain weight/g | 单株产量 Yield per plant /g |
|---|---|---|---|---|---|---|
| NT | 0.00 | 12.0±1.0 a | 100.7±11.2 a | 81.4±1.8 ab | 19.6±0.3 a | 19.1±0.1 b |
| 0.05 | 11.3±0.6 a | 105.5±4.4 b | 82.1±1.6 ab | 19.2±1.1 a | 18.8±1.3 b | |
| 0.10 | 12.3±0.6 a | 111.2±3.4 abc | 86.9±0.2 a | 19.4±1.1 a | 23.1±1.8 a | |
| 0.15 | 12.7±0.6 a | 121.0±5.2 a | 75.8±5.8 b | 18.6±0.8 a | 21.7±4.9 ab | |
| 0.30 | 12.3±1.5 a | 113.9±5.2 ab | 79.8±2.8 b | 19.1±0.8 a | 21.3±4.7 ab | |
| 0.50 | 13.3±1.5 a | 105.8±6.5 bc | 80.0±2.9 b | 19.1±0.7 a | 21.6±2.4 ab | |
| HT | 0.00 | 12.3±0.6 a | 52.5±0.9 d | 46.6±7.3 b | 18.0±1.0 a | 5.4±0.5 c |
| 0.05 | 13.0±2.0 a | 71.0±4.8 bc | 49.8±4.9 b | 17.6±0.3 a | 8.1±1.0 b | |
| 0.10 | 12.0±1.0 a | 83.2±3.1 a | 45.9±6.8 b | 17.7±0.2 a | 8.1±1.2 b | |
| 0.15 | 11.7±0.6 a | 85.9±4.2 a | 65.6±9.5 a | 17.7±0.3 a | 11.6±1.3 a | |
| 0.30 | 11.3±0.6 a | 76.5±1.6 b | 49.4±1.4 b | 18.1±0.5 a | 9.2±0.4 b | |
| 0.50 | 12.7±0.6 a | 65.7±4.7 c | 55.9±6.9 ab | 18.0±0.7 a | 8.4±1.6 b |
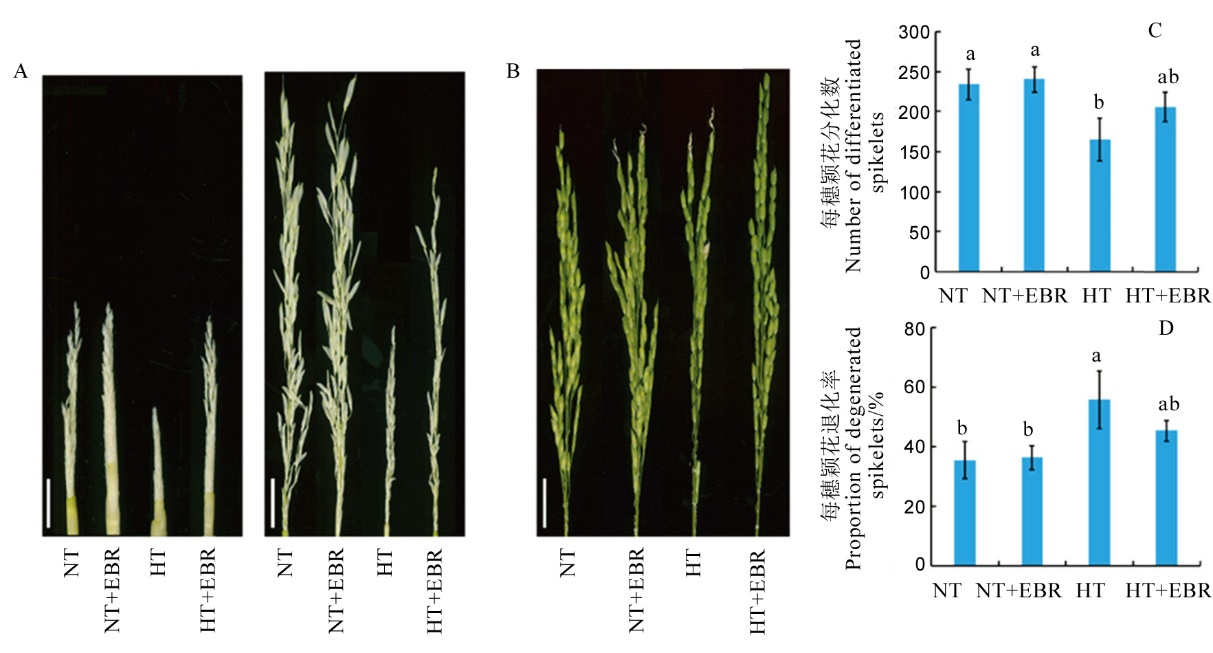
图1 高温下喷施EBR(0.15 mg/L)对幼穗发育及颖花形成的影响 A–高温及适温处理7 d和15 d的幼穗表型,标尺分别为0.5 cm和1 cm; B–高温和适温处理15 d水稻抽穗期穗部表型, 标尺为2.5 cm; C–0.15 mg/L EBR喷施对高温及适温下水稻颖花分化数的影响;D–0.15 mg/L EBR喷施对高温及适温下水稻颖花退化率的影响。数据为平均数±标准差。不同小写字母表示处理间差异显著(P<0.05)。下同。
Fig. 1. Effect of exogenous 2, 4-epibrassinolide (EBR) application(0.15 mg/L) on young panicle development and spikelet formation under high temperature. A, Young panicle under high temperature and normal temperature for 7 days (bar=0.5 cm) and 15 days(bar=1 cm); B, Panicle morphologies at heading stage at high temperature and normal temperature for 15 days(bar=2.5 cm); C, Effect of 0.15 mg/L exogenous EBR on the number of differentiated spikelets; D, Effect of 0.15 mg/L exogenous EBR application on the proportion of degenerated spikelets. Values are Mean±SD; Bars superscripted by different lowercase letters are significantly different at 0.05 level among treatments. The same as below.
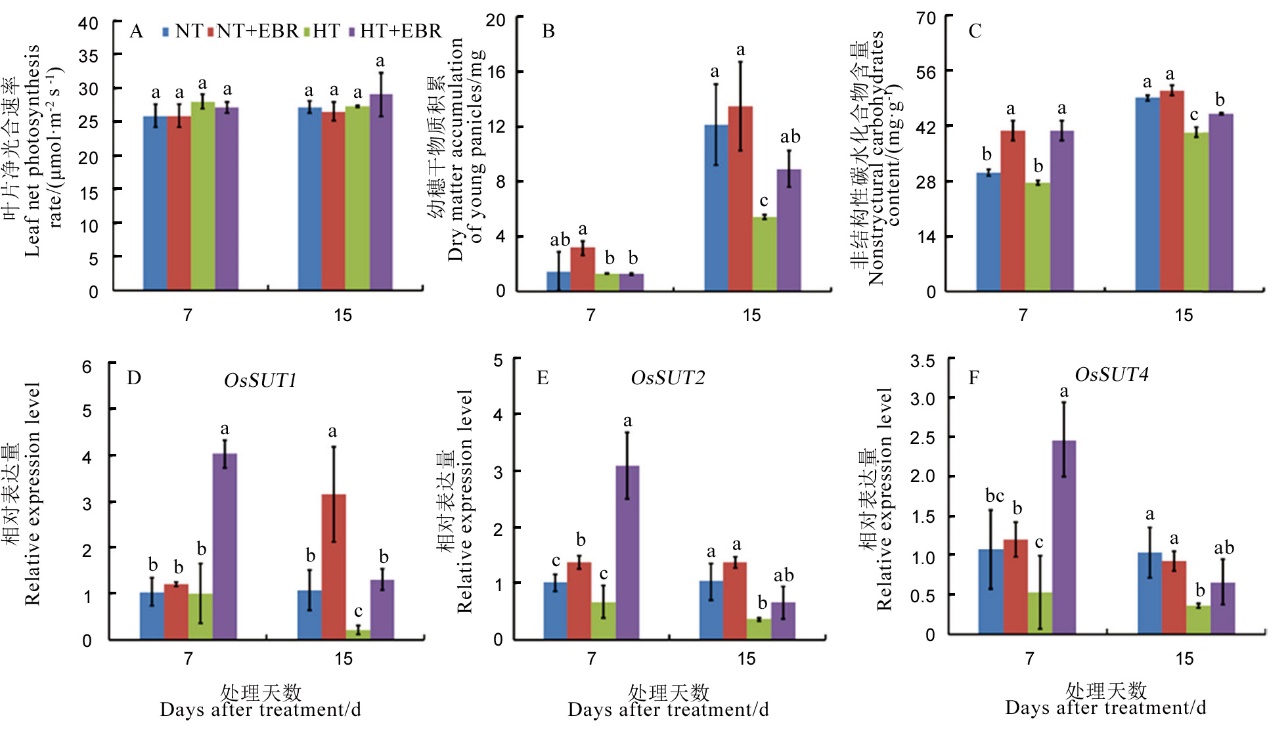
图2 高温下喷施EBR (0.15 mg/L)对幼穗中碳水化合物分配及转运的影响 A–顶部全展叶净光合速率; B–幼穗干物质积累; C–非结构性碳水化合物含量; D–OsSUT1相对表达量; E–OsSUT2相对表达量; F–OsSUT4相对表达量。
Fig. 2. Effect of 0.15 mg/L exogenous 2, 4-epibrassinolide (EBR) application on carbohydrate distribution and transportation of young panicles under high temperature. A, Leaf net photosynthesis; B, Dry matter accumulation of young panicles; C, Non-structural carbohydrate content; D, Relative expression of OsSUT1 of young panicles; E, Relative expression of OsSUT2 of young panicles; F, Relative expression of OsSUT4 of young panicles.
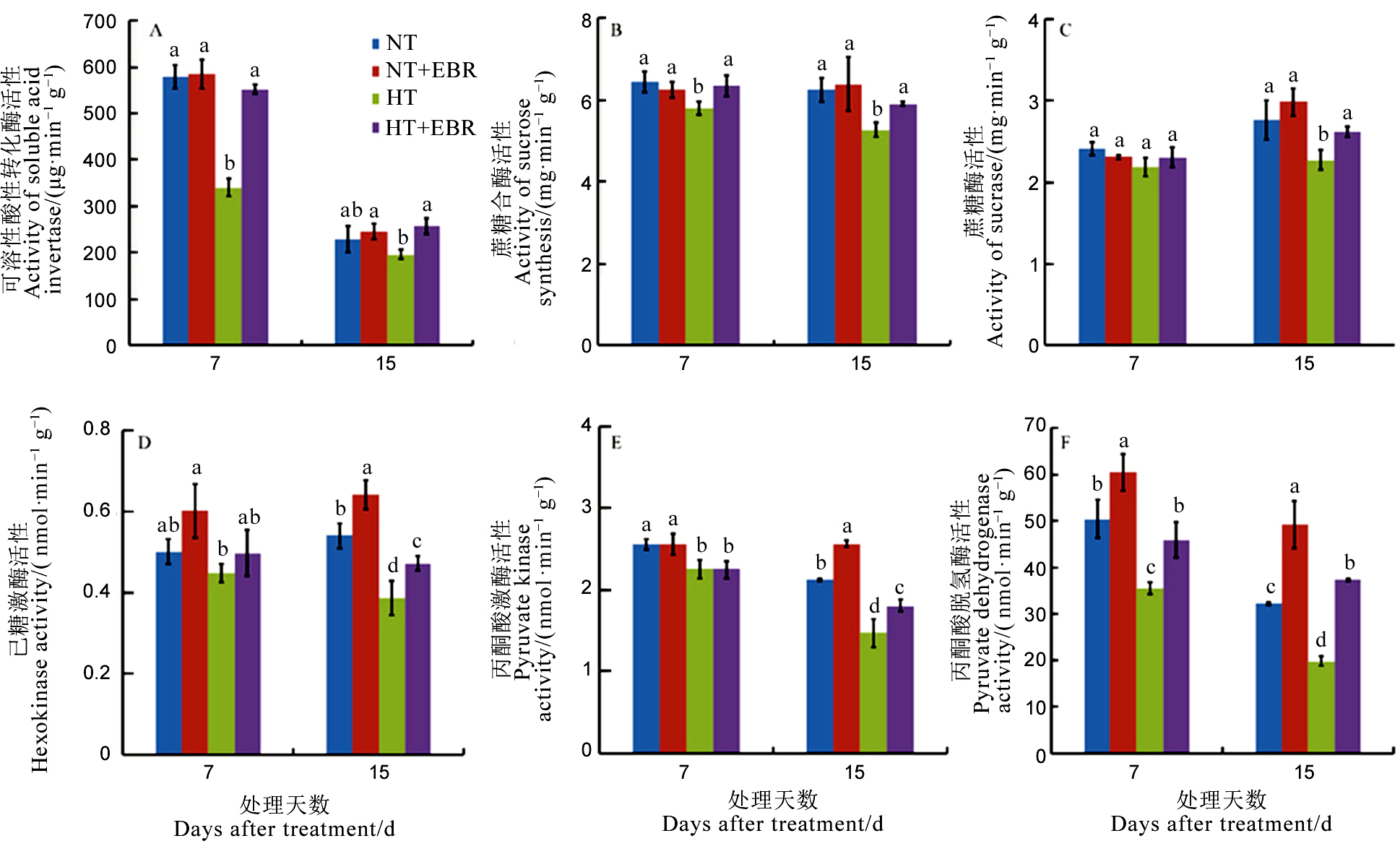
图3 高温下喷施EBR (0.15 mg/L) 喷施对幼穗中蔗糖利用相关酶活性的影响
Fig. 3. Effect of 0.15 mg/L exogenous 2, 4-epibrassinalide (EBR) application on enzymes activities related to sucrose utilization under high temperature.
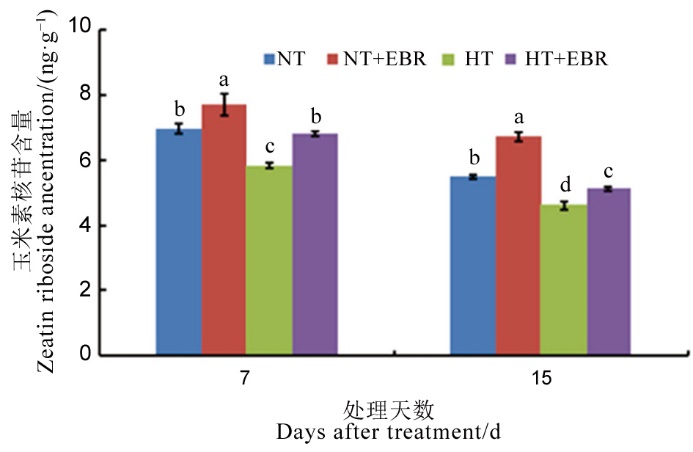
图4 高温下喷施EBR对幼穗玉米素核苷含量的影响
Fig. 4. Effect of 2, 4-epibrassinolide (EBR) application on zeatin riboside content in young panicles under high temperature.
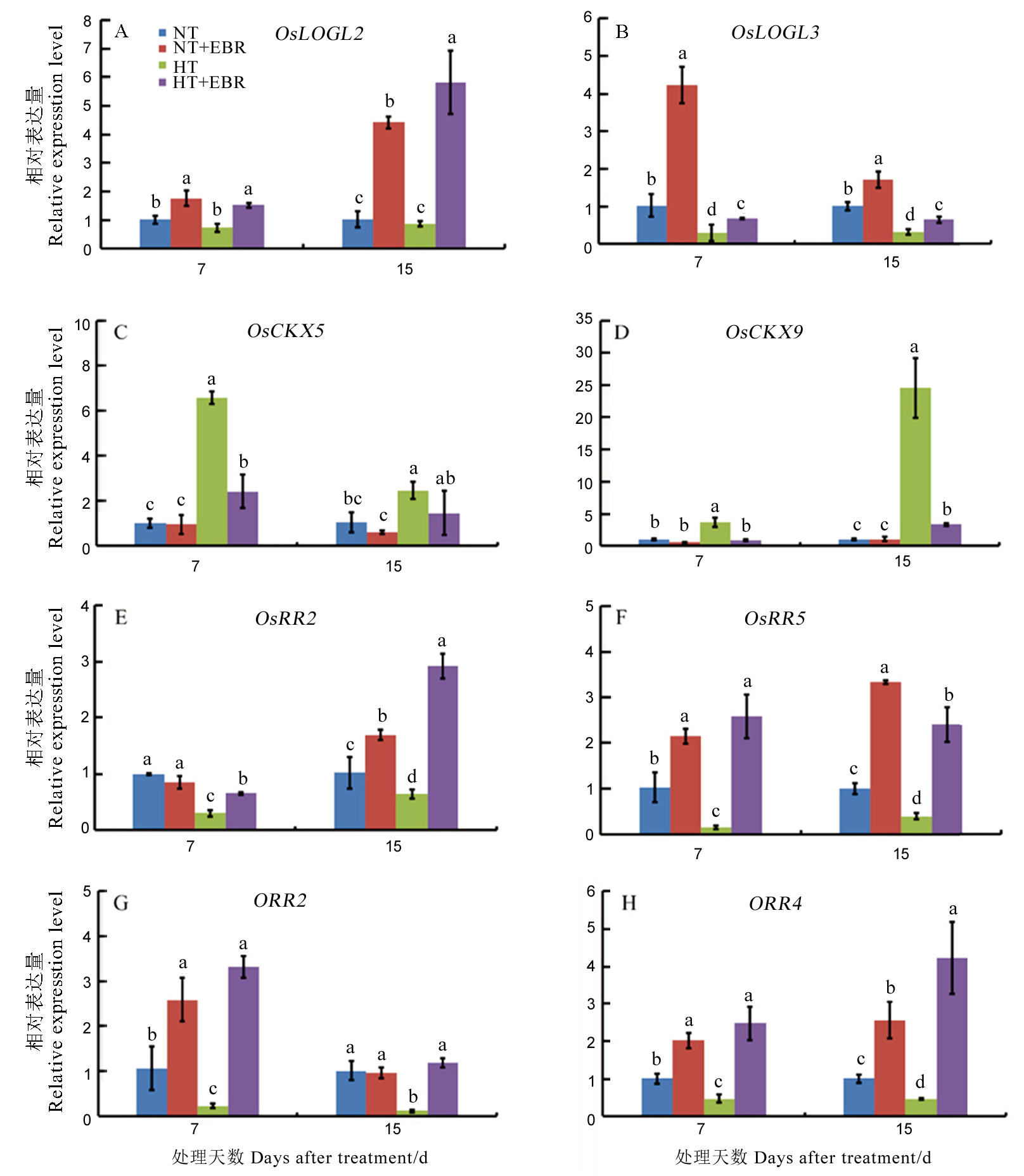
图5 高温下喷施EBR (0.15 mg/L)对幼穗中细胞分裂素相关代谢基因表达的影响
Fig. 5. Effect of 0.15 mg/L EBR application on relative expression of cytokinin metabolism genes under high temperature.
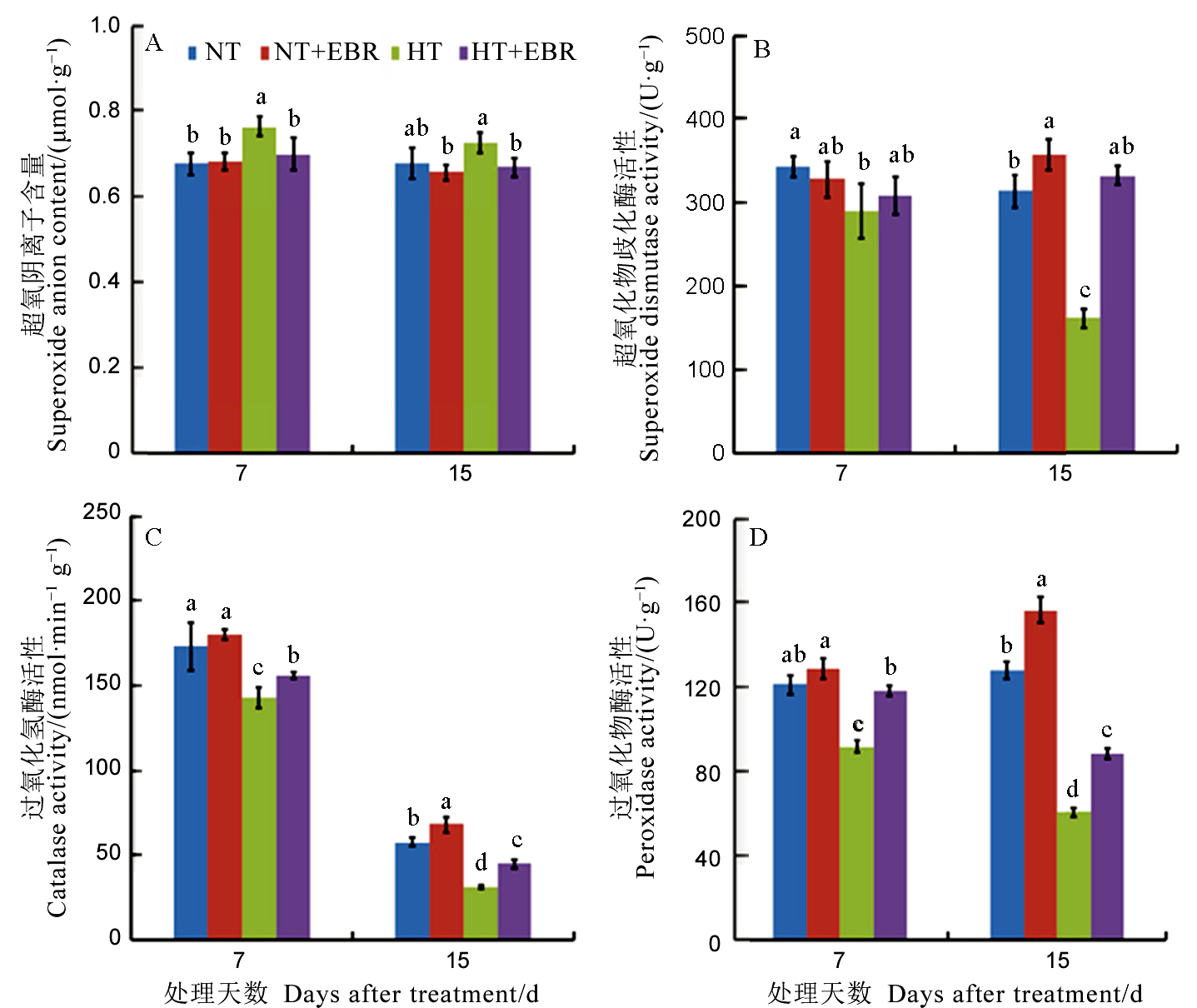
图6 高温下喷施EBR (0.15 mg/L) 对幼穗抗氧化能力的影响
Fig. 6. Effect of 0.15 mg/L exogenous 2, 4-epibrassinolide (EBR) application on antioxidant capacity of young panicles.
| [1] | Sanchez B, Rasmussen A, Porter J R.Temperatures and the growth and development of maize and rice: A review. Glob Chan Biol, 2014, 20: 408-417. |
| [2] | Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Peng S.Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice.Sci Rep, 2016: 6. |
| [3] | Wang Y, Wang L, Zhou J, Hu S, Chen H, Xiang J, Zhang Y, Zeng Y, Shi Q, Zhu D, Zhang Y,.Research progress on heat stress of rice at flowering.Rice Sci, 2019, 26: 1-10. |
| [4] | 柳新伟, 孟亚利, 周治国, 曹卫星. 水稻颖花分化与退化的动态特征. 作物学报, 2005, 31: 451-455. |
| Liu X W, Meng Y L, Zhou Z G, Gao W X.Dynamic characteristics of floret differentiation and degeneration in rice.Acta Agron Sin, 2005, 31: 451-455. (in Chinese with English abstract) | |
| [5] | Zhang C, Feng B, Chen T, Fu W, Li H, Li G, Jin Q, Tao L, Fu G.Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation.Environ Exp Bot, 2018, 155: 718-733. |
| [6] | Perdomo J A, Capo-Bauca S, Carmo-Silva E, Galmes J.Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, Wheat, and maize under high temperature and water deficit.Front Plant Sci, 2017: 8. |
| [7] | Takehara K, Murata K, Yamaguchi T, Yamaguchi K, Chaya G, Kido S, Iwasaki Y, Ogiwara H, Ebitani T, Miura K.Thermo-responsive allele of sucrose synthase3 (Sus3) provides high-temperature tolerance during the ripening stage in rice(Oryza sativa L.). Breeding Sci, 2018, 63: 336-342. |
| [8] | Miyazaki M, Araki M, Okamura K, Iwaya-Inoue M.Assimilate translocation and expression of sucrose transporter,OsSUT1, contribute to high-performance ripening under heat stress in the heat-tolerant rice cultivar Genkitsukushi. J Plant Physiol, 2013, 170: 1579-1584. |
| [9] | 丁承强. 氮素穗肥调控水稻每穗颖花数的分子机制. 南京: 南京农业大学, 2012. |
| Ding C Q.Molecular mechanism of nitrogen fertilization in increasing the spikelet number per panicle of rice. Nanjing: Nanjing Agricultural University, 2012. | |
| [10] | Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Mohapatra P K, Peng S.Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice.Front Plant Sci, 2017:8. |
| [11] | Vriet C, Russinova E, Reuzeau C.Boosting crop yields with plant steroids.Plant Cell, 2012, 24: 842-857. |
| [12] | Yu J Q, Huang L F, Hu W H, Zhou Y H, Mao W H, Ye S F, Nogues S.A role for brassinosteroids in the regulation of photosynthesis inCucumis sativus. J Exp Bot, 2004, 55: 1135-1143. |
| [13] | Zhang M, Zhai Z, Tian X, Duan L, Li Z.Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul, 2008, 56: 257-264. |
| [14] | Ryu H, Cho Y.Plant hormones in salt stress tolerance.J Plant Biol, 2015, 58: 147-155. |
| [15] | 松岛省三.稻作的理论与技术. 庞城译, 北京: 农业出版社, 1966: 121-133. |
| Matsushima S.Theory and Technology of Rice Cultivation. Pang C Trans.Beijing:Agriculture Press, 1966: 121-133. (in Chinese) | |
| [16] | Hansen J, Møller I.Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone.Anal Biochem, 1975, 68(1): 87-94. |
| [17] | 王杰, 王全, 田娜, 王瑜, 王爱香, 张克中, 崔金腾. 不同植物组织RNA提取方法的比较分析. 北京农学院学报, 2015, 30(1): 76-80. |
| Wang J, Wang Q, Tian N, Wang Y, Wang A X, Zhang K Z, Cui J T.Comparison and analysis of RNA extracting method from different plant tissues.J Beijing Univ Agric, 2015, 30(1): 76-80. | |
| [18] | Czechowski T, Bari R P, Stitt M, Scheible W R, Udvardi M K.Real-time RT-PCR profiling of over 1400Arabidopsis transcription factors: Unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J, 2004, 38: 366-379. |
| [19] | Wu C Y, Trieu A, Radhakrishnan P, Kwok S F, Harris S, Zhang K, Wang J, Wan J, Zhai H, Fujioka S, Feldmann K A, Pennell R I.Brassinosteroids regulate grain filling in rice.Plant Cell, 2008, 20: 2130-2145. |
| [20] | Wang F, Zhang Y, Guo Q, Tan H, Han J, Lin H, Wei H, Xu G, Zhu C.Effects of exogenous 5-aminolevulinic acid and 2,4-epibrassinolide on Cd accumulation in rice from Cd-contaminated soil.Rice Sci, 2018, 25: 320-329. |
| [21] | Clouse S D.Brassinosteroid/abscisic acid Antagonism in balancing growth and stress.Dev Cell, 2016, 38: 118-120. |
| [22] | 李赞堂, 王士银, 姜雯宇, 张帅, 张少斌, 徐江. 穗分化期外施24-表油菜素内酯(EBR)促进水稻源、库及籽粒灌浆的生理机制. 作物学报, 2018, 44(4): 581-590. |
| Li Z, Wang S, Jiang W, Zhang S, Zhang S, Xu J.Physiological mechanisms of promoting source, sink, and grain filling by 24-epibrassinolide (EBR) applied at panicle initiation stage of rice.Acta Agron Sin, 2018, 44(4): 581-590. (in Chinese with English abstract) | |
| [23] | Zhang C, Fu G, Yang X, Yang Y, Zhao X, Chen T, Jin Q, Tao L X.Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity.J Agron Crop Sci, 2016, 202: 394-408. |
| [24] | 杨洪建, 杨连新, 黄建晔, 刘红江, 董桂春, 颜士敏, 朱建国, 王余龙. FACE对武香粳14颖花分化与退化的影响. 作物学报, 2006, 32: 1076-1082. |
| Yang H, Yang L, Huang J, Liu H, Dong G, Yan S, Zhu J, Wang Y.Effect of free-air CO2 enrichment on spikelet differentiation and degeneration ofjaponica rice Wuxiangjing 14. Acta Agron Sin, 2006, 32: 1076-1082. (in Chinese with English abstract) | |
| [25] | Wang Z, Zhang W, Yang J.Physiological mechanism underlying spikelet degeneration in rice.J Integr Agric, 2018, 17: 1475-1481. |
| [26] | Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles E R, Qian Q, Kitano H, Matsuoka M.Cytokinin oxidase regulates rice grain production.Science, 2005, 309: 741-745. |
| [27] | Yuldashev R, Avalbaev A, Bezrukova M, Khripach V, Shakirova F.Cytokinin oxidase is involved in the regulation of cytokinin content by 24-epibrassinolide in wheat seedlings.Plant Physiol Biochem, 2012, 55: 1-6. |
| [28] | Hu Y, Bao F, Li J.Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway inArabidopsis. Plant J Cell & Mol Biol, 2010, 24: 693-701. |
| [29] | Ahmad P, Jaleel C A, Salem M A, Nabi G, Sharma S.Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress.Crit Rev Biotechnol, 2010, 30: 161-175. |
| [30] | 曹云英, 赵华. 高温胁迫下油菜素内酯对水稻幼苗的保护作用. 中国水稻科学, 2007, 21(5): 525-529. |
| Cao Y Y, Zhao H.Protective roles of brassinolide in rice seedling under heat stress.Chin J Rice Sci, 2007, 21(5): 525-529. (in Chinese with English abstract) | |
| [31] | Xia X, Fang P, Guo X, Qian X, Zhou J, Shi K, Zhou Y, Yu J.Brassinosteroid-mediated apophatic H2O2-glutare doxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato.Plant Cell Environ, 2017, 41: 1052-1064. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 赵艺婷, 谢可冉, 高逖, 崔克辉. 水稻分蘖期干旱锻炼对幼穗分化期高温下穗发育和产量形成的影响[J]. 中国水稻科学, 2024, 38(3): 277-289. |
| [12] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [13] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [14] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [15] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||