
中国水稻科学 ›› 2016, Vol. 30 ›› Issue (3): 247-255.DOI: 10.16819/j.1001-7216.2016.5172
王军1,2, 朱金燕2, 周勇1, 杨杰2, 范方军2, 李文奇2, 王芳权2, 仲维功2, 梁国华1,*( )
)
收稿日期:2015-11-18
修回日期:2016-01-07
出版日期:2016-05-10
发布日期:2016-05-10
通讯作者:
梁国华
基金资助:
Jun WANG1,2, Jin-yan ZHU2, Yong ZHOU1, Jie YANG2, Fang-jun FAN2, Wen-qi LI2, Fang-quan WANG2, Wei-gong ZHONG2, Guo-hua LIANG1,*( )
)
Received:2015-11-18
Revised:2016-01-07
Online:2016-05-10
Published:2016-05-10
Contact:
Guo-hua LIANG
摘要:
以广陆矮4号为受体,日本晴为供体的85个染色体单片段代换系群体为试验材料,通过单因素方差分析和Dunnett多重比较,测验单片段代换系与广陆矮4号之间抽穗期的差异,对代换片段上抽穗期相关的QTL进行了定位。以P≤0.001为阈值,在南京和海南不同温光条件下共定位到40个抽穗期相关的QTL。其中,21个QTL在2个环境中均被检测到;15个QTL只在南京环境中被检测到;4个QTL只在海南环境中被检测到。南京环境中定位到的36个抽穗期相关QTL,其加性效应值变化范围为2.8 d~15.7 d,加性效应百分率变化范围为3.8%~21.1%;海南环境中定位到的25个抽穗期相关QTL,加性效应值变化范围为1.8 d~12.1 d,加性效应百分率变化范围为1.7%~11.3%。这些QTL的定位,为进一步精细定位并克隆相应主效QTL和优异品种特定环境下的生育期改良奠定了基础。
中图分类号:
王军, 朱金燕, 周勇, 杨杰, 范方军, 李文奇, 王芳权, 仲维功, 梁国华. 不同温光条件下水稻抽穗期QTL的定位与分析[J]. 中国水稻科学, 2016, 30(3): 247-255.
Jun WANG, Jin-yan ZHU, Yong ZHOU, Jie YANG, Fang-jun FAN, Wen-qi LI, Fang-quan WANG, Wei-gong ZHONG, Guo-hua LIANG. QTL Analysis for Heading Date in Rice (Oryza sativa L.) Under Different Temperatures and Light Intensities[J]. Chinese Journal OF Rice Science, 2016, 30(3): 247-255.
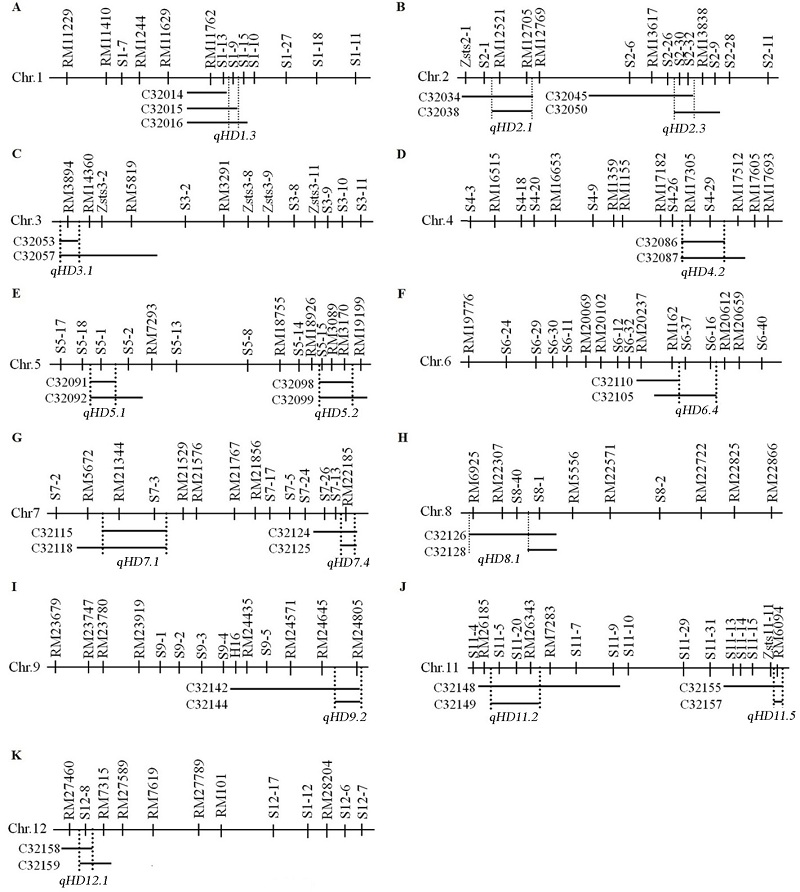
图2 水稻抽穗期QTL的代换作图粗黑线段为供体的代换片段,QTLs位于两条竖线之间。
Fig. 2. Substitution mapping of QTLs for the heading date in rice. The substituted segments are represented by horizontal dark bars. The regions to which the substituted segments best map QTLs are shown by two vertical dotted lines.
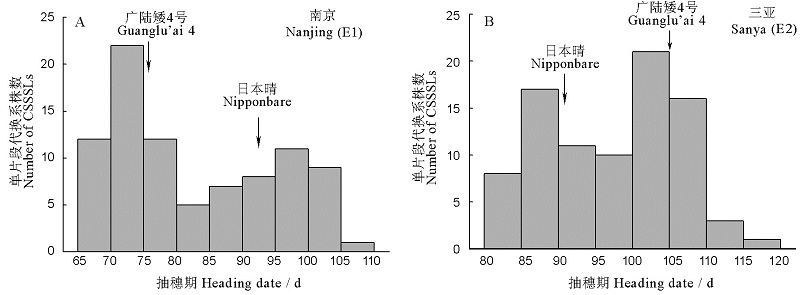
图1 染色体单片段代换系在不同环境中的抽穗期表型值分布
Fig. 1. Distribution of heading date of the Chromosome single segment substitution lines CSSSLs in the environments E1 and E2.
| 代换系 CSSSLs | 代换区间 Substituted segment | 长度 Length /Mb | 染色体 Chromosome | QTL | E1 | E2 | 类型 Type | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 表型 Phenotype | 加型效应 Additive effect/d | 加性效应百分率 Additive effect contribution/% | 表型 Phenotype | 加型效应 Additive effect/d | 加性效应百分率 Additive effect contribution/% | ||||||
| C32007 | RM6324-S1-3 | 7.34 | 1 | qHD1.1 | 67.0 | -4.6 | -6.0 | 84.6 | -10.3 | -9.8 | Ⅰ |
| C32009 | Lsts1-34-RM11189 | 7.92 | 1 | qHD1.2 | 68.3 | -4.0 | -5.2 | 85.4 | -9.9 | -9.4 | Ⅰ |
| C32016 | RM11762-S1-15 | 0.69 | 1 | qHD1.3 | 66.0 | -5.1 | -6.7 | 82.4 | -11.4 | -10.8 | Ⅰ |
| C32042 | RM13034-RM13263 | 8.67 | 2 | qHD2.2 | 70.0 | -3.3 | -4.3 | 85.8 | -9.9 | -9.4 | Ⅰ |
| C32050 | S2-30-S2-9 | 1.01 | 2 | qHD2.3 | 69.3 | -3.7 | -4.8 | 84.8 | -10.4 | -9.8 | Ⅰ |
| C32076 | S4-2 | 2.50 | 4 | qHD4.1 | 68.2 | -2.8 | -3.8 | 85.8 | -8.5 | -8.3 | Ⅰ |
| C32121 | RM21767-S7-17 | 5.83 | 7 | qHD7.2 | 69.2 | -4.4 | -5.8 | 85.2 | -10.9 | -10.2 | Ⅰ |
| C32122 | S7-5 | 1.48 | 7 | qHD7.3 | 66.0 | -6.0 | -7.9 | 82.8 | -12.1 | -11.3 | Ⅰ |
| C32129 | RM22722 | 1.39 | 8 | qHD8.2 | 70.4 | -3.8 | -5.0 | 86.6 | -10.2 | -9.5 | Ⅰ |
| C32145 | RM25213-S10-24 | 4.44 | 10 | qHD10.1 | 67.4 | -4.4 | -5.8 | 84.8 | -10.2 | -9.7 | Ⅰ |
| C32146 | S10-27-RM25486 | 3.62 | 10 | qHD10.2 | 68.2 | -4.0 | -5.2 | 84.2 | -10.5 | -10.0 | Ⅰ |
| C32153 | S11-9-ZHsts11-43 | 7.15 | 11 | qHD11.3 | 67.4 | -4.4 | -5.8 | 84.6 | -10.3 | -9.8 | Ⅰ |
| C32163 | S12-17 | 0.80 | 12 | qHD12.2 | 69.6 | -3.3 | -4.3 | 86.6 | -9.3 | -8.8 | Ⅰ |
| C32070 | S3-9-S3-10 | 3.80 | 3.0 | qHD3.3 | 99.3 | 12.8 | 17.3 | 109.0 | 3.1 | 3 | Ⅱ |
| C32102 | RM7158-S6-18 | 1.50 | 6 | qHD6.1 | 94.2 | 10.0 | 13.5 | 112.2 | 4.5 | 4.4 | Ⅱ |
| C32107 | RM20069 | 0.77 | 6 | qHD6.3 | 95.2 | 10.5 | 14.2 | 117.2 | 7.0 | 6.8 | Ⅱ |
| C32034 | Zsts2-1-RM12705 | 3.81 | 2 | qHD2.1 | 96.4 | 9.9 | 12.9 | 99.6 | -3.0 | -2.8 | Ⅲ |
| C32086 | RM17305-S4-29 | 3.79 | 4 | qHD4.2 | 92.9 | 9.6 | 12.9 | 99.2 | -1.8 | -1.8 | Ⅲ |
| C32113 | RM20659-S6-40 | 2.24 | 6 | qHD6.5 | 85.3 | 5.6 | 7.5 | 98.8 | -2.2 | -2.1 | Ⅲ |
| C32124 | S7-26-RM22185 | 0.33 | 7 | qHD7.4 | 101.7 | 11.9 | 15.6 | 103.4 | -1.8 | -1.7 | Ⅲ |
| C32126 | RM6925-S8-1 | 2.55 | 8 | qHD8.1 | 99.0 | 10.5 | 13.9 | 103.2 | -1.9 | -1.8 | Ⅲ |
| C32020 | S1-15-S1-27 | 3.60 | 1 | qHD1.4 | 102.1 | 13.0 | 17.0 | 104.2 | - | - | Ⅳ |
| C32053 | RM3894 | 1.02 | 2 | qHD3.1 | 92.1 | 7.8 | 10.1 | 103.2 | - | - | Ⅳ |
| C32071 | S3-11-S3-12 | 5.22 | 3 | qHD3.4 | 90.8 | 8.5 | 11.5 | 101.6 | - | - | Ⅳ |
| C32098 | S5-15-RM3170 | 4.26 | 5 | qHD5.2 | 100.4 | 13.1 | 17.7 | 102.8 | - | - | Ⅳ |
| C32105 | S6-21-S6-7 | 3.06 | 6 | qHD6.2 | 88.9 | 7.4 | 9.9 | 104.8 | - | - | Ⅳ |
| C32112 | RM162-S6-16 | 3.08 | 6 | qHD6.4 | 102.2 | 14.0 | 18.9 | 105.4 | - | - | Ⅳ |
| C32115 | RM21344-S7-3 | 6.60 | 7 | qHD7.1 | 95.0 | 10.4 | 14.0 | 103.2 | - | - | Ⅳ |
| C32130 | RM22825-RM22905 | 4.32 | 8 | qHD8.3 | 91.7 | 6.9 | 9.0 | 109.2 | - | - | Ⅳ |
| C32133 | RM23175-RM23642 | 8.07 | 8 | qHD8.4 | 85.5 | 3.8 | 4.9 | 109.0 | - | - | Ⅳ |
| C32140 | S9-4 | 1.54 | 9 | qHD9.1 | 97.3 | 9.7 | 12.7 | 109.8 | - | - | Ⅴ |
| C32144 | RM24805 | 1.47 | 9 | qHD9.2 | 99.4 | 10.7 | 14.1 | 109.0 | - | - | Ⅳ |
| C32147 | RM26076-S11-4 | 2.17 | 11 | qHD11.1 | 86.8 | 5.3 | 7.0 | 105.8 | - | - | Ⅳ |
| C32154 | S11-29-S11-31 | 4.33 | 11 | qHD11.4 | 88.7 | 6.3 | 8.2 | 104.8 | - | - | Ⅳ |
| C32157 | RM6094 | 0.08 | 11 | qHD11.5 | 94.3 | 9.1 | 11.9 | 103.2 | - | - | Ⅳ |
| C32158 | RM27460-S12-8 | 0.56 | 12 | qHD12.1 | 97.1 | 10.5 | 13.7 | 108.2 | - | - | Ⅳ |
| C32061 | RM3434-Zsts3-8 | 7.20 | 3 | qHD3.2 | 72.3 | - | - | 87.2 | -7.8 | -7.6 | Ⅴ |
| C32088 | M17605-RM17693 | 2.51 | 4 | qHD4.3 | 70.0 | - | - | 86.4 | -8.4 | -8.1 | Ⅴ |
| C32091 | S5-1 | 1.70 | 5 | qHD5.1 | 70.2 | - | - | 86.6 | -8.3 | -8.0 | Ⅴ |
| C32149 | S11-5-RM26343 | 3.60 | 11 | qHD11.2 | 72.4 | - | - | 84.8 | -10.2 | -9.7 | Ⅴ |
表1 不同环境中染色体单片段代换系抽穗期QTL及其效应
Table 1 Detection of the QTL and their additive effects for heading date in various CSSSLs in various environments.
| 代换系 CSSSLs | 代换区间 Substituted segment | 长度 Length /Mb | 染色体 Chromosome | QTL | E1 | E2 | 类型 Type | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 表型 Phenotype | 加型效应 Additive effect/d | 加性效应百分率 Additive effect contribution/% | 表型 Phenotype | 加型效应 Additive effect/d | 加性效应百分率 Additive effect contribution/% | ||||||
| C32007 | RM6324-S1-3 | 7.34 | 1 | qHD1.1 | 67.0 | -4.6 | -6.0 | 84.6 | -10.3 | -9.8 | Ⅰ |
| C32009 | Lsts1-34-RM11189 | 7.92 | 1 | qHD1.2 | 68.3 | -4.0 | -5.2 | 85.4 | -9.9 | -9.4 | Ⅰ |
| C32016 | RM11762-S1-15 | 0.69 | 1 | qHD1.3 | 66.0 | -5.1 | -6.7 | 82.4 | -11.4 | -10.8 | Ⅰ |
| C32042 | RM13034-RM13263 | 8.67 | 2 | qHD2.2 | 70.0 | -3.3 | -4.3 | 85.8 | -9.9 | -9.4 | Ⅰ |
| C32050 | S2-30-S2-9 | 1.01 | 2 | qHD2.3 | 69.3 | -3.7 | -4.8 | 84.8 | -10.4 | -9.8 | Ⅰ |
| C32076 | S4-2 | 2.50 | 4 | qHD4.1 | 68.2 | -2.8 | -3.8 | 85.8 | -8.5 | -8.3 | Ⅰ |
| C32121 | RM21767-S7-17 | 5.83 | 7 | qHD7.2 | 69.2 | -4.4 | -5.8 | 85.2 | -10.9 | -10.2 | Ⅰ |
| C32122 | S7-5 | 1.48 | 7 | qHD7.3 | 66.0 | -6.0 | -7.9 | 82.8 | -12.1 | -11.3 | Ⅰ |
| C32129 | RM22722 | 1.39 | 8 | qHD8.2 | 70.4 | -3.8 | -5.0 | 86.6 | -10.2 | -9.5 | Ⅰ |
| C32145 | RM25213-S10-24 | 4.44 | 10 | qHD10.1 | 67.4 | -4.4 | -5.8 | 84.8 | -10.2 | -9.7 | Ⅰ |
| C32146 | S10-27-RM25486 | 3.62 | 10 | qHD10.2 | 68.2 | -4.0 | -5.2 | 84.2 | -10.5 | -10.0 | Ⅰ |
| C32153 | S11-9-ZHsts11-43 | 7.15 | 11 | qHD11.3 | 67.4 | -4.4 | -5.8 | 84.6 | -10.3 | -9.8 | Ⅰ |
| C32163 | S12-17 | 0.80 | 12 | qHD12.2 | 69.6 | -3.3 | -4.3 | 86.6 | -9.3 | -8.8 | Ⅰ |
| C32070 | S3-9-S3-10 | 3.80 | 3.0 | qHD3.3 | 99.3 | 12.8 | 17.3 | 109.0 | 3.1 | 3 | Ⅱ |
| C32102 | RM7158-S6-18 | 1.50 | 6 | qHD6.1 | 94.2 | 10.0 | 13.5 | 112.2 | 4.5 | 4.4 | Ⅱ |
| C32107 | RM20069 | 0.77 | 6 | qHD6.3 | 95.2 | 10.5 | 14.2 | 117.2 | 7.0 | 6.8 | Ⅱ |
| C32034 | Zsts2-1-RM12705 | 3.81 | 2 | qHD2.1 | 96.4 | 9.9 | 12.9 | 99.6 | -3.0 | -2.8 | Ⅲ |
| C32086 | RM17305-S4-29 | 3.79 | 4 | qHD4.2 | 92.9 | 9.6 | 12.9 | 99.2 | -1.8 | -1.8 | Ⅲ |
| C32113 | RM20659-S6-40 | 2.24 | 6 | qHD6.5 | 85.3 | 5.6 | 7.5 | 98.8 | -2.2 | -2.1 | Ⅲ |
| C32124 | S7-26-RM22185 | 0.33 | 7 | qHD7.4 | 101.7 | 11.9 | 15.6 | 103.4 | -1.8 | -1.7 | Ⅲ |
| C32126 | RM6925-S8-1 | 2.55 | 8 | qHD8.1 | 99.0 | 10.5 | 13.9 | 103.2 | -1.9 | -1.8 | Ⅲ |
| C32020 | S1-15-S1-27 | 3.60 | 1 | qHD1.4 | 102.1 | 13.0 | 17.0 | 104.2 | - | - | Ⅳ |
| C32053 | RM3894 | 1.02 | 2 | qHD3.1 | 92.1 | 7.8 | 10.1 | 103.2 | - | - | Ⅳ |
| C32071 | S3-11-S3-12 | 5.22 | 3 | qHD3.4 | 90.8 | 8.5 | 11.5 | 101.6 | - | - | Ⅳ |
| C32098 | S5-15-RM3170 | 4.26 | 5 | qHD5.2 | 100.4 | 13.1 | 17.7 | 102.8 | - | - | Ⅳ |
| C32105 | S6-21-S6-7 | 3.06 | 6 | qHD6.2 | 88.9 | 7.4 | 9.9 | 104.8 | - | - | Ⅳ |
| C32112 | RM162-S6-16 | 3.08 | 6 | qHD6.4 | 102.2 | 14.0 | 18.9 | 105.4 | - | - | Ⅳ |
| C32115 | RM21344-S7-3 | 6.60 | 7 | qHD7.1 | 95.0 | 10.4 | 14.0 | 103.2 | - | - | Ⅳ |
| C32130 | RM22825-RM22905 | 4.32 | 8 | qHD8.3 | 91.7 | 6.9 | 9.0 | 109.2 | - | - | Ⅳ |
| C32133 | RM23175-RM23642 | 8.07 | 8 | qHD8.4 | 85.5 | 3.8 | 4.9 | 109.0 | - | - | Ⅳ |
| C32140 | S9-4 | 1.54 | 9 | qHD9.1 | 97.3 | 9.7 | 12.7 | 109.8 | - | - | Ⅴ |
| C32144 | RM24805 | 1.47 | 9 | qHD9.2 | 99.4 | 10.7 | 14.1 | 109.0 | - | - | Ⅳ |
| C32147 | RM26076-S11-4 | 2.17 | 11 | qHD11.1 | 86.8 | 5.3 | 7.0 | 105.8 | - | - | Ⅳ |
| C32154 | S11-29-S11-31 | 4.33 | 11 | qHD11.4 | 88.7 | 6.3 | 8.2 | 104.8 | - | - | Ⅳ |
| C32157 | RM6094 | 0.08 | 11 | qHD11.5 | 94.3 | 9.1 | 11.9 | 103.2 | - | - | Ⅳ |
| C32158 | RM27460-S12-8 | 0.56 | 12 | qHD12.1 | 97.1 | 10.5 | 13.7 | 108.2 | - | - | Ⅳ |
| C32061 | RM3434-Zsts3-8 | 7.20 | 3 | qHD3.2 | 72.3 | - | - | 87.2 | -7.8 | -7.6 | Ⅴ |
| C32088 | M17605-RM17693 | 2.51 | 4 | qHD4.3 | 70.0 | - | - | 86.4 | -8.4 | -8.1 | Ⅴ |
| C32091 | S5-1 | 1.70 | 5 | qHD5.1 | 70.2 | - | - | 86.6 | -8.3 | -8.0 | Ⅴ |
| C32149 | S11-5-RM26343 | 3.60 | 11 | qHD11.2 | 72.4 | - | - | 84.8 | -10.2 | -9.7 | Ⅴ |

图3 多态性标记的物理位置及40个抽穗期QTL在水稻12条染色体上的分布染色体右侧为分子标记名称, 左侧数字代表物理位置(单位Mb)。黑色区段显示19个新抽穗期相关QTL所在的位置范围, 灰色区段显示与前人研究具有相同区段的21个抽穗期相关QTL所在的位置。
Fig. 3. Physical locations of polymorphic markers and the distribution of 40 QTLs for heading date on 12 rice chromosomes. The molecular markers are indicated in the right side, and the physical locations (Mb) of each marker are indicated in the left side. The regions of 19 newly detected QTLs for heading date are shown in black, and the regions of the other 21 QTLs which share the same regions with the previously detected QTLs are shown in gray.
| [1] | Chang T T, Li C C, Vergara B S.Component analysis of duration from seeding to heading in rice by the basic vegetative phase and the photoperiod-sensitive phase.Euphytica, 1969, 18(1): 79-91. |
| [2] | Tsai K H.Gene loci and alleles controlling the duration of basic vegetative growth of rice.Rice Genet, 1986, 5(6): 339-349. |
| [3] | 胡时开, 苏岩, 叶卫军, 等. 水稻抽穗期遗传与分子调控机理研究进展. 中国水稻科学, 2012, 26(3): 373-382. |
| Hu S K, Su Y, Ye W J, et al.Advances in genetic analysis and molecular regulation mechanism of heading date in rice (Oryza sativa L.).Chin J Rice Sci, 2012, 26(3): 189-196. (in Chinese with English abstract) | |
| [4] | Yano M, Katayose Y, Ashikari M, et al.Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the arabidopsis flowering time gene constans.Plant Cell, 2000, 12(12): 2473-2483. |
| [5] | Itoh H, Nonoue Y, Yano M, et al.A pair of floral regulators sets critical day length for Hd3a florigen expression in rice.Nat Genet, 2010, 42(7): 635-638. |
| [6] | Takahashi Y, Shomura A, Sasaki T, et al.Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the a subunit of protein kinase CK2.Proc Natl Acad Sci USA, 2001, 98(14): 7922-7927. |
| [7] | Hori K, Ogiso-Tanaka E, Matsubara K, et al.Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response.Plant J, 2013, 76(1): 36-46. |
| [8] | Matsubara K, Ogiso-Tanaka E, Hori K, et al.Natural variation in Hd17, a homolog of arabidopsis ELF3 that is involved in rice photoperiodic flowering.Plant Cell Physiol, 2012, 53(4): 709-716. |
| [9] | Doi K, Izawa T, Fuse T, et al.Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd.Genes Dev, 2004, 18(8): 926-936. |
| [10] | Matsubara K, Yamanouchi U, Wang Z X, et al.Ehd2, a rice ortholog of the maize INDETERMINATE 1 gene, promotes flowering by up-regulating Ehd.Plant Physiol, 2008, 148(3): 1425-1435. |
| [11] | Matsubara K, Yamanouchi U, Nonoue Y, et al.Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering.Plant J, 2011, 66(4): 603-612. |
| [12] | Gao H, Zheng X M, Fei G, et al.Ehd4 encodes a novel and Oryza-genus-specific regulator of hotoperiodic flowering in rice.PLoS Genet, 2013, 9(2): e1003281. |
| [13] | Andrés F, Galbraith D W, Talón M, et al.Analysis of photoperiod sensitivity sheds light on the role of phytochromes in photoperiodic flowering in rice.Plant Physiol, 2009, 151(2): 681-690. |
| [14] | Xue W Y, Xing Y Z, Weng X Y, et al.Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice.Nat Genet, 2008, 40(6): 761-767. |
| [15] | Yan W H, Liu H Y, Zhou X C, et al.Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice.Cell Res, 2013, 23(7): 969-971. |
| [16] | Wu W X, Zheng X M, Lu G W, et al.Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia.Proc Natl Acad Sci USA, 2013, 110(8): 2775-2780. |
| [17] | Wei X J, Xu J F, Guo H N, et al.DTH8 Suppresses flowering in rice, influencing plant height and yield potential simultaneously.Plant Physiol, 2010, 153(4): 1747-1758. |
| [18] | 刘冠明, 李文涛, 曾瑞珍, 等. 水稻单片段代换系代换片段的QTL鉴定. 遗传学报, 2004, 31(12): 1395-1400. |
| Liu G M, Li W T, Zeng R Z, et al.Identification of QTLs on substituted segments in single segment substitution lines of rice.J Genet Genom, 2004, 31(12): 1395-1400. (in Chinese with English abstract) | |
| [19] | Eshed Y, Zamir D.An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL.Genetics, 1995, 141(3): 1147-1162. |
| [20] | McCouch S R,CGSNL.Gene nomenclature system for rice.Rice,2008,1(1): 72-84. |
| [21] | Paterson A H, Deverna J W, Lanini B, et al.Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes in an interspecies cross of tomato.Genetics, 1990, 124(3): 735-742. |
| [22] | Young N D, Tanksley S D.Restriction fragment length polymorphism maps and the concept of graphical genotypes.Theor Appl Genet, 1989, 77(1): 95-101. |
| [23] | Xu J J, Zhao Q, Du P N, et al.Developing high throughput genotyped chromosome segment substitution lines based on population whole-genome re-sequencing in rice (Oryza sativa L.).BMC Genom, 2010, 24(11): 656-669. |
| [24] | Shomura A, Izawa T, Ebana K, et al.Deletion in a gene associated with grain size increased yields during rice domestication.Nat Genet, 2008, 40(8): 1023-1028. |
| [25] | Li Y B, Fan C C, Xing Y Z, et al.Natural variation in GS5 plays an important role in regulating grain size and yield in rice.Nat Genet, 2011, 43(12): 1266-1269. |
| [26] | Shen G J, Xing Y Z.Two novel QTLs for heading date are identified using a set of chromosome segment substitution lines in rice (Oryza sativa L.).J Genet Genom, 2014, 41(12): 659-662. |
| [27] | 何风华, 席章营, 曾瑞珍, 等. 利用单片段代换系定位水稻抽穗期QTL. 中国农业科学, 2005, 38(8): 1505-1513. |
| He F H, Xi Z Y, Zeng R Z, et al.Mapping of heading date QTLs in rice (Oryza sativa L.) using single segment substitution lines.Sci Agric Sin, 2005, 38(8): 1505-1513. (in Chinese with English abstract) | |
| [28] | 杨德卫, 张亚东, 朱镇, 等. 基于CSSL的水稻抽穗期QTL定位及遗传分析. 植物学报, 2010, 45(2): 189-197. |
| Yang D W, Zhang Y D, Zhu Z, et al.Mapping and genetic analysis of quantitative trait loci for heading date with chromosome segment substitution lines in Oryza sativa.Chin Bull Bot, 2010, 45(2): 189-197. (in Chinese with English abstract) | |
| [29] | 周勇, 崔国昆, 张言周, 等. 水稻抽穗期主效QTL qHd8.1的精细定位. 中国水稻科学, 2012, 26(1): 43-48. |
| Zhou Y, Cui G K, Zhang Y Z, et al.Fine mapping of a major QTL qHd8.1 for heading date in rice.Chin J Rice Sci, 2012, 26(1): 43-48. (in Chinese with English abstract) | |
| [30] | 张永生, 江玲, 刘喜, 等. 控制水稻品种Koshihikari 抽穗期的数量性状位点. 作物学报, 2008, 34(11): 1869-1876. |
| Zhang Y S, Jiang L, Liu X, et al.Quantitative trait loci for rice heading time in Koshihikari.Acta Agron Sin, 2008, 34(11): 1869-1876. (in Chinese with English abstract) | |
| [31] | Cheng L R, Wang J M, Ye G Y, et al.Identification of stably expressed QTL for heading date using eciprocal introgression line and recombinant inbred line populations in rice. Genet Res (Camb), 2012, 94(5): 245-253. |
| [32] | Lee S, Jia M H, Jia Y L, et al.Tagging quantitative trait loci for heading date and plant height in important breeding parents of rice (Oryza sativa).Euphytica, 2014, 197(2): 191-200. |
| [33] | 曾晶, 姜恭好, 何予卿, 等. 利用籼粳交探讨水稻株高和抽穗期的遗传基础. 分子植物育种, 2006, 4(4): 527-534. |
| Zeng J, Jiang G H, He Y Q, et al.The genetic bases analysis of plant height and heading date in Indica/Japonica hybrids.Mol Plant Breeding, 2006, 4(4): 527-534. (in Chinese with English abstract) | |
| [34] | 邵迪, 李秋萍, 吴比, 等. 利用染色体片段代换系定位水稻主效抽穗期QTL. 湖南农业大学学报: 自然科学版: 2009, 35(4): 344-347. |
| Shao D, Li Q P, Wu B, et al.Mapping of a major QTL for heading date in rice using chromosome segment substitution lines. J Hunan Agric Univ:Nat Sci, 2009, 35(4): 344-347. (in Chinese with English abstract) | |
| [35] | 冯跃, 翟荣荣, 曹立勇, 等. 不同施氮水平下水稻株高与抽穗期的QTL比较分析. 作物学报, 2011, 37(9): 1525-1532. |
| Feng Y, Zhai R R, Cao L Y, et al.QTL analysis for plant height and heading date in rice under two nitrogen levels.Acta Agron Sin, 2011, 37(9): 1525-1532. (in Chinese with English abstract) | |
| [36] | Wang B B, Zhu C X, Liu X, et al.Fine mapping of qHD4-1, a QTL controlling the heading date, to a 20.7-kb DNA fragment in rice (Oryza sativa L.).Plant Mol Biol Rep, 2011, 29(3): 702-713. |
| [37] | Pei C G, Liu X, Wang W Y, et al.Fine mapping of qHD8-1, a QTL controlling the heading date, to a 26-kb DNA fragment in rice (Oryza sativa L.).J Plant Biol, 2011, 54(3): 190-198. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||