
中国水稻科学 ›› 2021, Vol. 35 ›› Issue (2): 112-120.DOI: 10.16819/j.1001-7216.2021.0602
郑亚莉1, 余林闯1, 安肖肖1, 程心乐1, 任丽君1, 苏子龙1, 郑小雅1, 兰涛1,2,3,*( )
)
收稿日期:2020-06-02
修回日期:2020-09-04
出版日期:2021-03-10
发布日期:2021-03-10
通讯作者:
兰涛
基金资助:
Yali ZHENG1, Linchuang YU1, Xiaoxiao AN1, Xinle CHENG1, Lijun REN1, Zilong SU1, Xiaoya ZHENG1, Tao LAN1,2,3,*( )
)
Received:2020-06-02
Revised:2020-09-04
Online:2021-03-10
Published:2021-03-10
Contact:
Tao LAN
摘要:
【目的】水稻OsWOX3B基因调控叶片形态和表皮毛发育,根据表型被命名为LSY1、DEP、NUDA和GLR1等。深入了解OsWOX3B基因对水稻发育调控的功能具有重要意义。【方法】利用CRISPR/Cas9基因编辑技术对籼稻品种R401的OsWOX3B进行基因敲除。对所获材料进行突变位点分析和表型分析,同时进行相关基因的表达分析。【结果】所获材料的OsWOX3B基因的编码区第341位碱基由T变为C,第395–397位碱基缺失,其叶片和颖壳光滑,与突变体dep、nuda、glr1的表型相同,可确认其为Oswox3b突变体。除了与已报道的OsWOX3B的功能缺失突变表型相关外,Oswox3b突变体也有新表型出现。与野生型R401相比,突变体Oswox3b表现为生育期延长、分蘖数减少、叶片变宽、稻穗变长和每穗粒数增多。同时,突变体Oswox3b的剑叶维管束增多,小维管束间距增大,2个水稻侧生器官发育相关基因在突变体Oswox3b中的表达变化也与其表型变化一致。【结论】鉴定了一个新的水稻OsWOX3B基因突变体,其影响水稻侧生器官发育。
郑亚莉, 余林闯, 安肖肖, 程心乐, 任丽君, 苏子龙, 郑小雅, 兰涛. 一份水稻OsWOX3B基因敲除突变体的鉴定[J]. 中国水稻科学, 2021, 35(2): 112-120.
Yali ZHENG, Linchuang YU, Xiaoxiao AN, Xinle CHENG, Lijun REN, Zilong SU, Xiaoya ZHENG, Tao LAN. Identification of a Knockout Mutant of OsWOX3B Gene in Rice (Oryza sativa L.)[J]. Chinese Journal OF Rice Science, 2021, 35(2): 112-120.
| 再生株系 | 阳性株系 | 阳性率 | 编辑株系 | 编辑率 | 纯合株系 | 杂合株系 |
|---|---|---|---|---|---|---|
| Regenerated lines | Positive lines | Positive rate/% | Edited lines | Editing rate/% | Homozygous lines | Heterozygous lines |
| 18 | 18 | 100 | 12 | 66.7 | 2 | 10 |
表1 水稻OsWOX3B基因敲除T0代株系分子鉴定结果
Table 1 Molecular identification results of OsWOX3B knockout T0 generation lines.
| 再生株系 | 阳性株系 | 阳性率 | 编辑株系 | 编辑率 | 纯合株系 | 杂合株系 |
|---|---|---|---|---|---|---|
| Regenerated lines | Positive lines | Positive rate/% | Edited lines | Editing rate/% | Homozygous lines | Heterozygous lines |
| 18 | 18 | 100 | 12 | 66.7 | 2 | 10 |
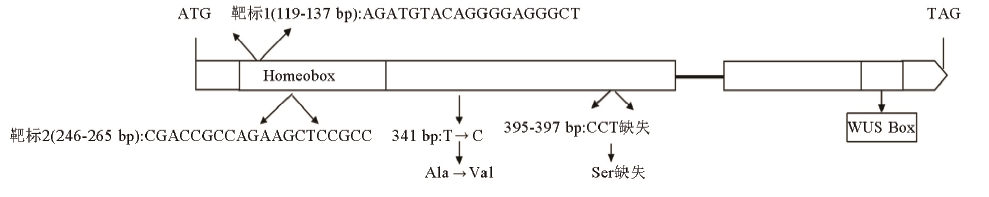
图1 水稻OsWOX3B基因敲除株系的编辑位点方框表示外显子,线段表示内含子,箭头表示突变位点和结构域。
Fig. 1. Editing sites of OsWOX3B knockout line. Box represents exon, line segment represents intron and arrow represents mutation site and domain.
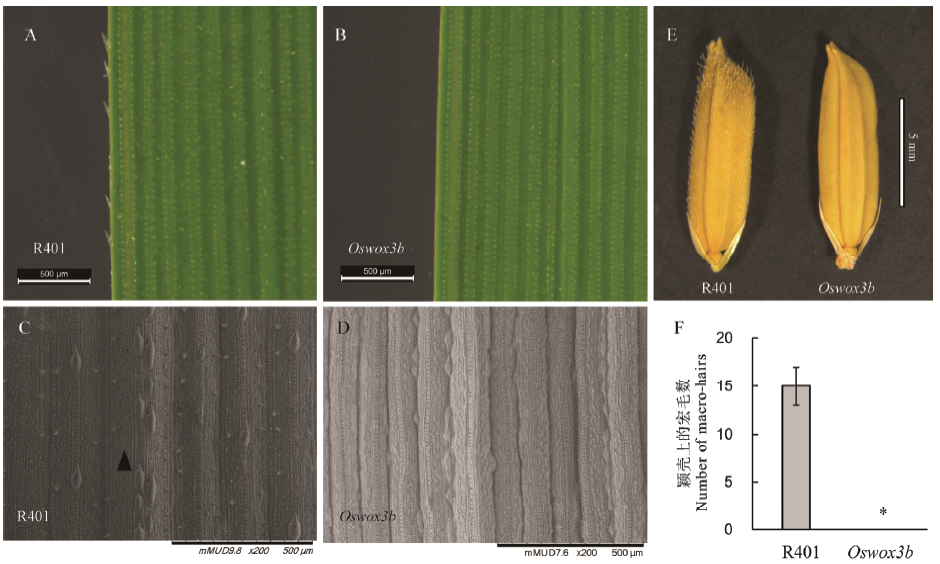
图2 突变体Oswox3b叶片和颖壳上表皮毛数目分析 A、B和E表示野生型R401和纯合突变株分蘖期叶片及成熟种子体式显微镜观察结果;C、D表示野生型R401和纯合突变株分蘖期叶片扫描电镜观察结果,箭头所指为宏毛。F-R401和oswox3b近轴面叶中部宏毛数目统计结果。柱形图上的数据点为平均值±标准差(n=3)。*表示突变体在P<0.05水平差异显著。
Fig. 2. Analysis of the number of epidermal hairs on the leaf and seed glume of Oswox3b mutant. A, B and E show the microscopic observation results of leaf and mature seed of wild-type R401 and homozygous mutants in tillering stage; C and D show the tillering stage leaf observation results in wild-type R401 and homozygous mutants by SEM (the arrow: macro-hair). F shows the macro-hair number in the middle adaxial surface of wild type R401 and oswox3b. The data points on the chart are mean ± standard deviation (n=3). *shows the difference is significant at P < 0.05 level.
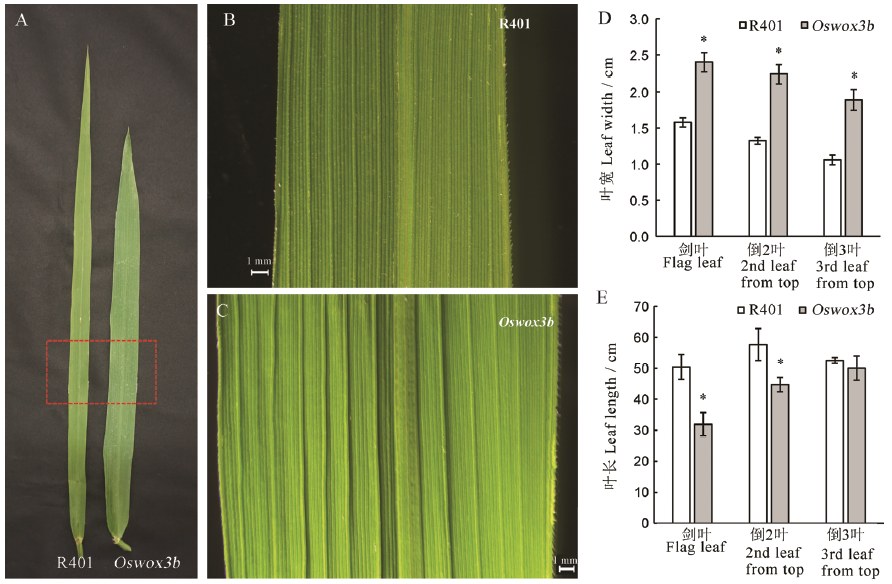
图3 突变体Oswox3b的叶长和叶宽 A-抽穗期野生型R401与突变体Oswox3b的剑叶;B,C-A中红色区域;D-抽穗期野生型R401与突变体Oswox3b的叶宽;E-抽穗期野生型R401与突变体Oswox3b的叶长。柱形图上的数据点为平均值±标准差(n=10); *表示突变体在P<0.05水平上差异显著。
Fig. 3. Leaf length and leaf width of mutant Oswox3b. A, Wild type R401 and mutant Oswox3b at heading stage; B, C, The red area in A under a body microscope; D, Leaf width of wild type R401 and mutant Oswox3b at heading stage; E, Leaf length of wild type R401 and mutant Oswox3b at heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference is significant at P < 0.05 level.
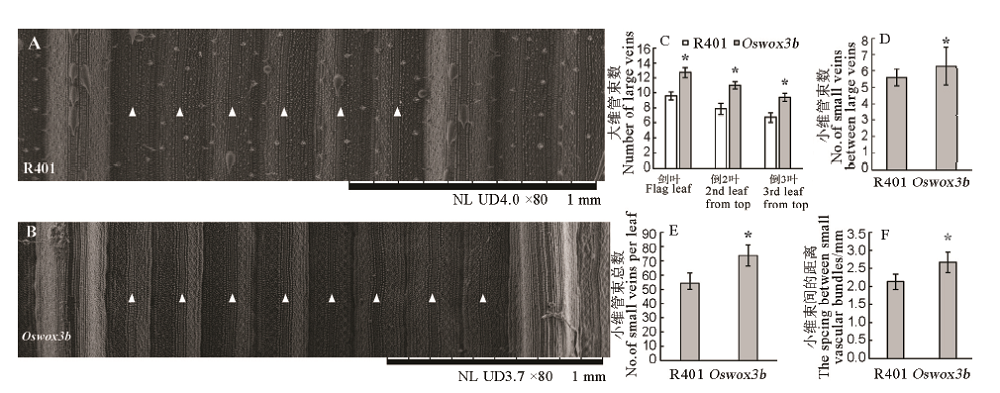
图4 突变体Oswox3b叶片维管束分析 A、B-抽穗期野生型(A)与突变体(B)叶片大维管束之间小维管束扫描电镜观察结果,白色三角形代表小维管束;C-抽穗期大维管束数目统计分析;D-抽穗期剑叶大维管束之间小维管束数目;E-抽穗期剑叶小维管束总数;F-抽穗期剑叶小维管束间的距离。柱形图上的数据点为平均值±标准差(n=10);*表示突变体在P<0.05水平差异显著。
Fig. 4. Analysis of vascular bundles in leaves of mutant Oswox3b. A and B, Small vascular bundle between the wild type (A) and the mutant (B) leaf at heading stage by SEM. White triangle represents small vascular bundle; C, Large vascular bundle quantity at heading stage; D, Small vascular bundle quantity between the large vascular bundles of the flag leaf at heading stage; E, Total small vascular bundle quantity of the flag leaf at heading stage; F, Spacing between small vascular bundles of the flag leaf at heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference was significant at P < 0.05 level.
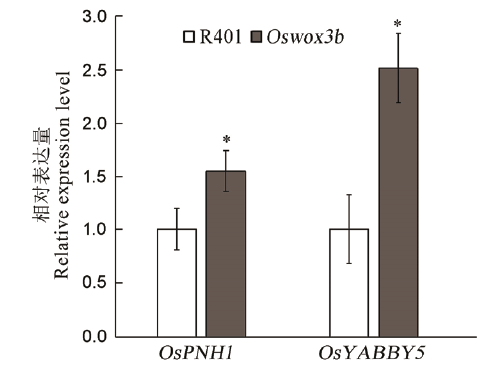
图5 突变体Oswox3b影响了维管束发育相关基因的表达柱形图上的数据点为平均值±标准差(n=3),*表示突变体在P<0.05水平差异显著。
Fig. 5. The mutant Oswox3b affected the expression of genes related to vascular bundle development. The data points on the column chart are mean value ± standard deviation (n=3); *shows the difference was significant at P < 0.05 level.
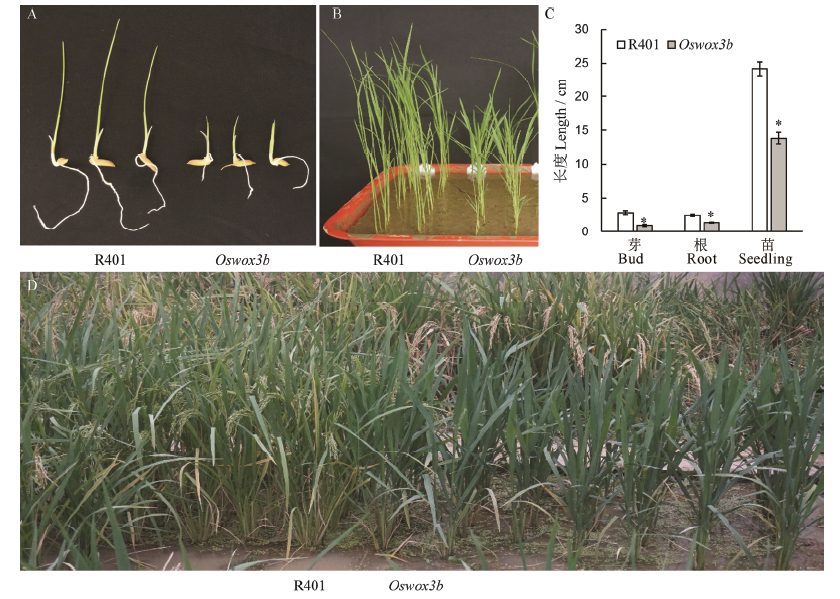
图6 野生型R401与突变体Oswox3b不同生育期表型比较 A和B分别表示芽期和幼苗期野生型与突变体;C-芽长、根长和幼苗株高;D-抽穗期野生型与突变体植株的田间表型。柱形图上的数据点为平均值±标准差(n=10),*表示突变体在P<0.05水平差异显著。
Fig. 6. Comparison of phenotypes between wild type R401 and mutant Oswox3b at various growth stages. A, B, Phenotypes of wild type and mutant in bud stage and seedling stage, respectively; C, Bud length, root length and plant height in A and B; D, Field phenotype of wild type and mutant plants in heading stage. The data points on the column chart are mean value ± standard deviation (n=10); *shows the difference was significant at P < 0.05 level.
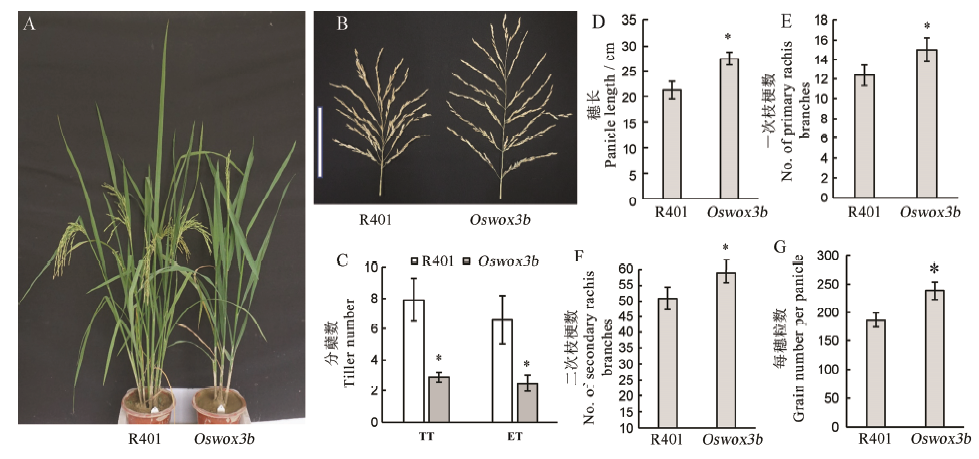
图7 突变体Oswox3b的分蘖、穗型和千粒重 A-抽穗期野生型R401与突变体Oswox3b的植株表型;B-野生型R401与突变体Oswox3b穗形比较(比例尺为15 cm);C-抽穗期野生型R401与突变体Oswox3b分蘖数目和株高,B中TT为总分蘖数,ET为有效分蘖数;D~G分别为野生型与突变体穗长,一次枝梗数,二次枝梗数和每穗总粒数的统计分析。柱形图上的数据点为平均值±标准差(n=10),*表示突变体在P<0.05水平差异显著。
Fig. 7. Tiller, panicle and 1000-grain weight of mutant Oswox3b. A, Plant phenotype of wild type and mutant at heading stage; B, Comparison of panicle shape between wild type R401 and mutant Oswox3b (scale: 15 cm); C, Statistical analysis of tiller number and plant height of wild type R401 and mutant Oswox3b; D–G, Statistical analysis of panicle length, primary rachis branch number, secondary rachis branch number and total grain number of wild type and mutant, respectively. The data points on the column chart are mean value ± standard deviation (n=10); *Means the difference was significant at P < 0.05 level.
| [1] | 矫永庆. 水稻理想株型基因Ideal Plant Architecture 1(IPA1)的克隆与功能研究[D]. 北京: 中国科学院, 2010. |
| Jiao Y Q.Cloning and functional study of ideal plant architecture 1(IPA1)[D]. Beijing: Chinese Academy of Sciences, 2010. (in Chinese with English abstract). | |
| [2] | 于燕杰, 张大兵, 袁政. WOX蛋白家族调控干细胞发育分子机制的研究进展[J]. 植物学报, 2016, 51(4): 565-574. |
| Yu Y J, Zhang D B, Yuan Z.Progress in the molecular mechanism of WOX protein family regulating stem cell development[J]. Chinese Bulletin of Botany, 2016, 51(4): 565-574. (in Chinese with English abstract) | |
| [3] | 高丽, 孙祎敏, 邵铁梅, 孔卫娜, 崔润丽, 卢楠, 仵陶. 植物WUSCHEL-related homeobox (WOX) 家族研究进展[J]. 生物技术通报, 2015, 31(5): 7-12. |
| Gao L, Sun W M, Shao T M, Kong W N, Cui R L, Lu N, Wu T.Research progress of WUSCHEL related homeobox (WOX) family of plants[J]. Biotechnology Bulletin, 2015, 31(5): 7-12. (in Chinese with English abstract) | |
| [4] | Zhao Y, Hu Y, Dai M, Huang L, Zhou D X.The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice[J]. Plant Cell, 2009, 21(3): 736-748. |
| [5] | Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou D X.The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling[J]. Plant Cell, 2015, 27(9): 2469-2483. |
| [6] | Chen G, Feng H, Hu Q, Qu H, Chen A, Yu L, Xu G.Improving rice tolerance to potassium deficiency by enhancing OsHAK16p: WOX11-controlled root development[J]. Plant Biotechnology Journal, 2015, 13(6): 833-848. |
| [7] | Kamiya N, Nagasaki H, Morikami A, Sato Y, Matsuoka M.Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem[J]. Plant Journal, 2003, 35(4): 429-441. |
| [8] | Chu H W, Liang W Q, Li J, Hong F, Wu Y F, Wang L K, Wang J, Wu P, Liu C M, Zhang Q F, Xu J, Zhang D B.A CLE-WOX signaling module regulates root meristem maintenance and vascular tissue development in rice[J]. Journal of Experimental Botany, 2013, 64: 5359-5369. |
| [9] | Wang W F, Li G, Zhao J, Chu H W, Lin W H, Zhang D B, Wang Z Y, Liang W Q.DWARF TILLER1, a WUSCHEL-related homeobox transcription factor, is required for tiller growth in rice[J/OL]. PLoS Genetics, 2014, 10: e1004154. |
| [10] | Lu Z F, Shao G N, Xiong J S, Jiao Y Q, Wang J, Liu G F, Meng X B, Liang Y, Xiong G S, Wang Y H, Li J Y.MONOCULM 3, an ortholog of WUSCHEL in rice, is required for tiller bud formation[J]. Journal of Genetics and Genomics, 2015, 42(2): 71-78. |
| [11] | Yasui Y, Ohmori Y, Takebayashi Y, Sakakibara H, Hirano H Y.WUSCHEL-RELATED HOMEOBOX4 acts as a key regulator in early leaf development in rice[J/OL].PLoS Genetics, 2018, 14: e1007365. |
| [12] | Cho S H, Kang K, Lee S H, Lee I J, Paek N C.OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa)[J]. Journal of Experimental Botany, 2016, 67(6): 1677-1687. |
| [13] | Cho S H, Yoo S C, Zhang H, Pandeya D, Koh H J, Hwang J Y, Kim G T, Paek N C.The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development[J]. New Phytologist, 2013, 198(4): 1071-1084. |
| [14] | Dai M, Hu Y, Zhao Y, Liu H, Zhou D X.A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development[J]. Plant Physiology, 2007, 144(1): 380-390. |
| [15] | Obara M, Ikeda K, Itoh J I, Nagato Y.Characterization of leaf lateral symmetry 1 mutant[J]. Breeding Science, 2004, 54(2): 157-163. |
| [16] | Honda E, Yew C L, Yoshikawa T, Sato Y, Hibara K I, Itoh J I.LEAF LATERAL SYMMETRY1, a member of the WUSCHEL-RELATED HOMEOBOX3 gene family, regulates lateral organ development differentially from other paralogs, NARROW LEAF2 and NARROW LEAF3 in rice[J]. Plant Cell Physiology, 2018, 59(2): 376-391. |
| [17] | Angeles-Shim R B, Asano K, Takashi T, Shim J, Kuroha T, Ayano M, Ashikari M. A WUSCHEL-related homeobox 3B gene, depilous (dep), confers glabrousness of rice leaves and glumes[J]. Rice, 2012, 5(1): 28. |
| [18] | Zhang H L, Wu K, Wang Y F, Peng Y, Hu F Y, Wen L, Han B, Qian Q, Teng S.A WUSCHEL-like homeobox gene, OsWOX3B responses to NUDA/GL-1 locus in rice[J]. Rice, 2012, 5(1): 30. |
| [19] | Li J, Yuan Y, Lu Z, Yang L, Gao R, Lu J, Li J, Xiong G.Glabrous rice 1, encoding a homeodomain protein, regulates trichome development in rice[J]. Rice, 2012, 5(1): 32. |
| [20] | Sun W Q, Gao D W, Xiong Y, Tang X X, Xiao X F, Wang C G, Yu S B.Hairy Leaf 6, an AP2/ERF transcription factor, interacts with OsWOX3B and regulates trichome formation in rice[J]. Molecular Plant, 2017, 10(11): 1417-1433. |
| [21] | Nishimura A, Ito M, Kamiya N, Sato Y, Matsuoka M.OsPNH1 regulates leaf development and maintenance of the shoot apical meristem in rice[J]. Plant Journal, 2002, 30(2): 189-201. |
| [22] | Tanaka W, Toriba T, Ohmori Y, Yoshida A, Kawai A, Mayama-Tsuchida T, Ichikawa H, Mitsuda N, Ohme-Takagi M, Hirano H Y.The YABBY gene TONGARI-BOUSHI1 is involved in lateral organ development and maintenance of meristem organization in the rice spikelet[J]. Plant Cell, 2012, 24(1): 80-95. |
| [23] | Ishiwata A, Ozawa M, Nagasaki H, Kato M, Noda Y, Yamaguchi T, Nosaka M, Shimizu-Sato S, Nagasaki A, Maekawa M, Hirano H Y, Sato Y.Two WUSCHEL-related homeobox genes, narrow leaf 2 and narrow leaf 3, control leaf width in rice[J]. Plant and Cell Physiology, 2013, 54(5): 779-792. |
| [24] | Chen M, Luo J, Shao G, Wei X, Tang S, Sheng Z, Song J, Hu P.Fine mapping of a major QTL for flag leaf width in rice, qFLW4, which might be caused by alternative splicing of NAL1[J]. Plant Cell Reports, 2012, 31(5): 863-872. |
| [25] | Qi J, Qian Q, Bu Q, Li S, Chen Q, Sun J, Liang W, Zhou Y, Chu C, Li X, Ren F, Palme K, Zhao B, Chen J, Chen M, Li C.Mutation of the rice narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport[J]. Plant Physiology, 2008, 147(4): 1947-1959. |
| [26] | Zhang X, Zong J, Liu J L, Yin J Y, Zhang D B.Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar[J]. Journal of Integrative Plant Biology, 2010, 52(11): 1016-1026. |
| [27] | Yoo S C, Cho S H, Paek N C. Rice WUSCHEL-related homeobox 3A (OsWOX3A) modulates auxin-transport gene expression in lateral root and root hair development[J/OL]. Plant Signaling and Behavior, 2013, 8(10): 10, e25929. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||