
中国水稻科学 ›› 2025, Vol. 39 ›› Issue (5): 679-689.DOI: 10.16819/j.1001-7216.2025.241014
丁国华1,3,#, 李鑫2,#, 曹良子1, 周劲松1, 雷蕾1, 白良明1, 洛育1, 杨光1, 崔志波3, 赵明辉3,*( ), 孙世臣1,*(
), 孙世臣1,*( )
)
收稿日期:2024-10-31
修回日期:2025-03-25
出版日期:2025-09-10
发布日期:2025-09-10
通讯作者:
*email: mhzhao@syau.edu.cn;email: sunshichen1979@163.com
作者简介:#共同第一作者
基金资助:
DING Guohua1,3,#, LI Xin2,#, CAO Liangzi1, ZHOU Jinsong1, LEI Lei1, BAI Liangming1, LUO Yu1, YANG Guang1, CUI Zhibo3, ZHAO Minghui3,*( ), SUN Shichen1,*(
), SUN Shichen1,*( )
)
Received:2024-10-31
Revised:2025-03-25
Online:2025-09-10
Published:2025-09-10
Contact:
*email: mhzhao@syau.edu.cn;email: sunshichen1979@163.com
摘要: 【目的】解析龙稻18(LD18)和龙稻17029(L9)孕穗期光合系统对低温反应差异,为培育孕穗期耐冷高产水稻品种提供理论依据。【方法】以优质耐冷品种LD18和其与旱稻创制的高产冷敏感新种质L9为试验材料,利用人工气候室研究了孕穗期低温冷害对不同耐冷材料剑叶叶绿体超微结构、光合参数、相关酶活性及基因表达的影响。【结果】测序分析表明,LD18和L9基因组相似程度为75.03%,大的差异片段主要在2号、5号染色体。孕穗期低温下,LD18空壳率显著低于L9,LD18剑叶叶色值和叶绿体超微结构变化不明显,L9叶色值显著降低,叶绿体和类囊体出现膨大、变形,并产生了大量嗜锇粒,LD18光合相关酶活性高于L9。转录组及RT-qPCR结果表明,LD18差异表达基因数多于L9;GO和KEGG分析显示,LD18和L9相比最富集的基因通路包括光合作用和叶绿素代谢; RT-qPCR分析结果表明光合相关基因RBCX1在LD18中上调表达3倍,而在L9中下调表达。【结论】孕穗期低温下,LD18比L9具有更强的耐冷性,LD18能够调动更多基因应对低温胁迫,保持叶绿体结构完整,不降低叶绿素含量及光合速率,光合相关酶活性更高。
丁国华, 李鑫, 曹良子, 周劲松, 雷蕾, 白良明, 洛育, 杨光, 崔志波, 赵明辉, 孙世臣. 孕穗期低温对寒地不同水稻材料光合系统的影响研究[J]. 中国水稻科学, 2025, 39(5): 679-689.
DING Guohua, LI Xin, CAO Liangzi, ZHOU Jinsong, LEI Lei, BAI Liangming, LUO Yu, YANG Guang, CUI Zhibo, ZHAO Minghui, SUN Shichen. Effect of Low Temperature at Booting Stage on Photosynthetic System of Different Rice Materials in Cold Region[J]. Chinese Journal OF Rice Science, 2025, 39(5): 679-689.
| 基因ID Gene ID | 序列 Sequence(5' to 3') |
|---|---|
| 18S-F | CTACGTCCCTGCCCTTTGTACA |
| 18S-F | ACACTTCACCGGACCATTCAA |
| LOC4327351-F | ATTAGCCATCAACGACCA |
| LOC4327351-R | ACTCTCCAATCTGCTCTTC |
| LOC4331490-F | TGGCACAACTTACCTCTG |
| LOC4331490-R | CGCACATCTTCTACATCCT |
| LOC4333690-F | AACTGGAGGAACTGATTGG |
| LOC4333690-R | CACTGGACACGATAACGA |
| LOC4335640-F | GTCCCTTTACGACAATGGT |
| LOC4335640-R | CACCCGTGCTATCCATTAG |
| LOC4345611-F | GAGGTCCGATCTGCTTATG |
| LOC4345611-R | GCTGGTCTCCAAGACATAG |
| LOC4352460-F | ATGGCTTGTAGAGTAATGCT |
| LOC4352460-R | CTCTGCTGATCTGAACTCAT |
表1 RT-qPCR所用引物
Table 1. Primers used for RT-qPCR in this study
| 基因ID Gene ID | 序列 Sequence(5' to 3') |
|---|---|
| 18S-F | CTACGTCCCTGCCCTTTGTACA |
| 18S-F | ACACTTCACCGGACCATTCAA |
| LOC4327351-F | ATTAGCCATCAACGACCA |
| LOC4327351-R | ACTCTCCAATCTGCTCTTC |
| LOC4331490-F | TGGCACAACTTACCTCTG |
| LOC4331490-R | CGCACATCTTCTACATCCT |
| LOC4333690-F | AACTGGAGGAACTGATTGG |
| LOC4333690-R | CACTGGACACGATAACGA |
| LOC4335640-F | GTCCCTTTACGACAATGGT |
| LOC4335640-R | CACCCGTGCTATCCATTAG |
| LOC4345611-F | GAGGTCCGATCTGCTTATG |
| LOC4345611-R | GCTGGTCTCCAAGACATAG |
| LOC4352460-F | ATGGCTTGTAGAGTAATGCT |
| LOC4352460-R | CTCTGCTGATCTGAACTCAT |
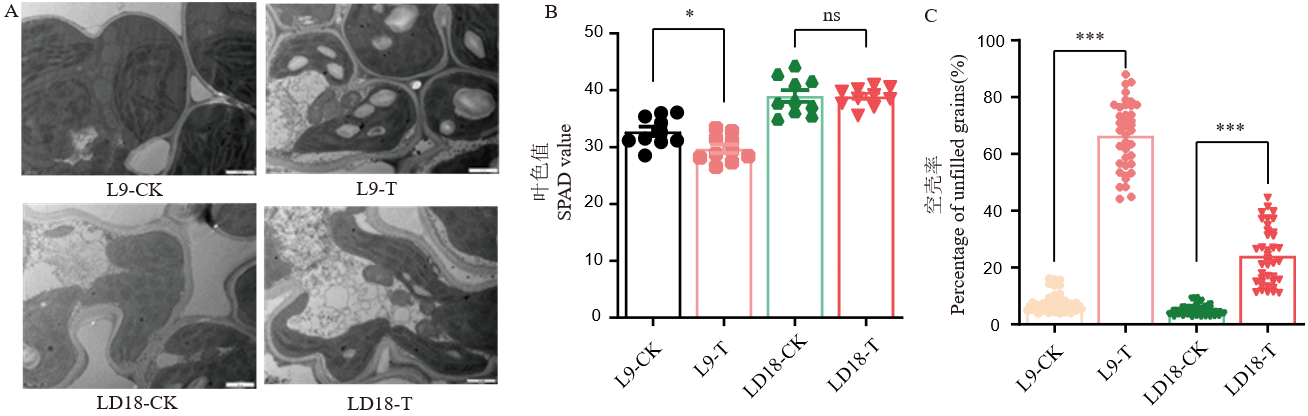
图2 孕穗期低温下LD18和L9剑叶叶绿体超微结构(A)、叶色值(B)及空壳率(C) ns:无显著差异; *:P<0.05;***:P<0.001。T表示低温处理(17℃);CK表示常温处理(25℃)。下同。
Fig. 2. Changes in chloroplast ultrastructure, SPAD value of flag leaves and percentage of unfilled grains for LD18 and L9 at low temperature during booting stage ns, No significant difference; *, P<0.05; ***, P<0.001.T, Chilling treatment((17℃), CK, Normal temperature treatment(25℃).
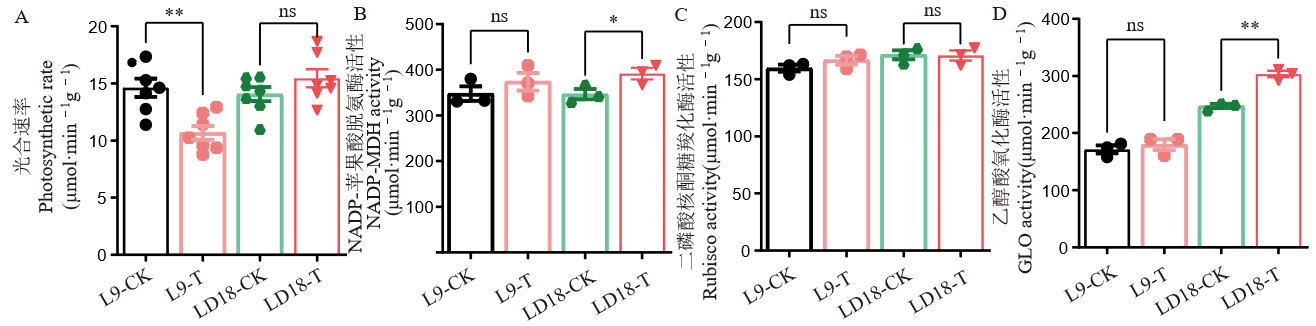
图3 孕穗期低温下LD18和L9剑叶光合速率及碳同化、能量代谢相关酶活性 ns:无显著差异; *:P<0.05;**:P<0.01。
Fig. 3. Activities of enzymes involved in carbon assimilation and energy metabolism in LD18 and L9 exposed to cold stress at the booting stage ns, No significant difference; *, P<0.05; **, P<0.01.
| 样品名称 Sample | GC含量 GC content (%) | ≥Q30百分比 ≥Q30 percent (%) | 比对片段 Comparison fragment (bp) | 比对到参考基因组 Compared to the percentage of the reference genome(%) |
|---|---|---|---|---|
| LD18-T1 | 52.39 | 94.46 | 41,811,440 | 95.93 |
| LD18-T2 | 52.81 | 94.30 | 42,371,082 | 95.66 |
| LD18-T3 | 52.59 | 94.42 | 41,630,423 | 95.60 |
| LD18-CK1 | 53.16 | 94.50 | 37,394,980 | 95.58 |
| LD18-CK2 | 53.06 | 94.53 | 43,242,371 | 95.01 |
| LD18-CK3 | 52.63 | 94.93 | 39,951,714 | 96.15 |
| L9-T1 | 52.76 | 94.89 | 37,865,138 | 95.43 |
| L9-T2 | 51.66 | 94.19 | 44,570,598 | 95.03 |
| L9-T3 | 52.62 | 92.88 | 41,324,819 | 94.96 |
| L9-CK1 | 53.03 | 93.87 | 36,590,085 | 94.88 |
| L9-CK2 | 52.84 | 93.92 | 40,478,494 | 95.25 |
| L9-CK3 | 52.52 | 93.29 | 41,705,157 | 94.54 |
表2 转录组测序数据统计
Table 2. Transcriptome sequencing data statistics
| 样品名称 Sample | GC含量 GC content (%) | ≥Q30百分比 ≥Q30 percent (%) | 比对片段 Comparison fragment (bp) | 比对到参考基因组 Compared to the percentage of the reference genome(%) |
|---|---|---|---|---|
| LD18-T1 | 52.39 | 94.46 | 41,811,440 | 95.93 |
| LD18-T2 | 52.81 | 94.30 | 42,371,082 | 95.66 |
| LD18-T3 | 52.59 | 94.42 | 41,630,423 | 95.60 |
| LD18-CK1 | 53.16 | 94.50 | 37,394,980 | 95.58 |
| LD18-CK2 | 53.06 | 94.53 | 43,242,371 | 95.01 |
| LD18-CK3 | 52.63 | 94.93 | 39,951,714 | 96.15 |
| L9-T1 | 52.76 | 94.89 | 37,865,138 | 95.43 |
| L9-T2 | 51.66 | 94.19 | 44,570,598 | 95.03 |
| L9-T3 | 52.62 | 92.88 | 41,324,819 | 94.96 |
| L9-CK1 | 53.03 | 93.87 | 36,590,085 | 94.88 |
| L9-CK2 | 52.84 | 93.92 | 40,478,494 | 95.25 |
| L9-CK3 | 52.52 | 93.29 | 41,705,157 | 94.54 |
| 样品 Sample | 差异表达基因数量 Number of DEGs | 上调基因数量 Up-regulated genes | 下调基因数量 Down-regulated genes |
|---|---|---|---|
| LD18-CK vs LD18-T | 4509 | 2243 | 2266 |
| L9-CK vs L9-T | 3093 | 1432 | 1661 |
表3 孕穗期低温下两个材料发生差异表达基因数量
Table 3. Number of differentially expressed genes of the two materials at booting stage under cold stress
| 样品 Sample | 差异表达基因数量 Number of DEGs | 上调基因数量 Up-regulated genes | 下调基因数量 Down-regulated genes |
|---|---|---|---|
| LD18-CK vs LD18-T | 4509 | 2243 | 2266 |
| L9-CK vs L9-T | 3093 | 1432 | 1661 |
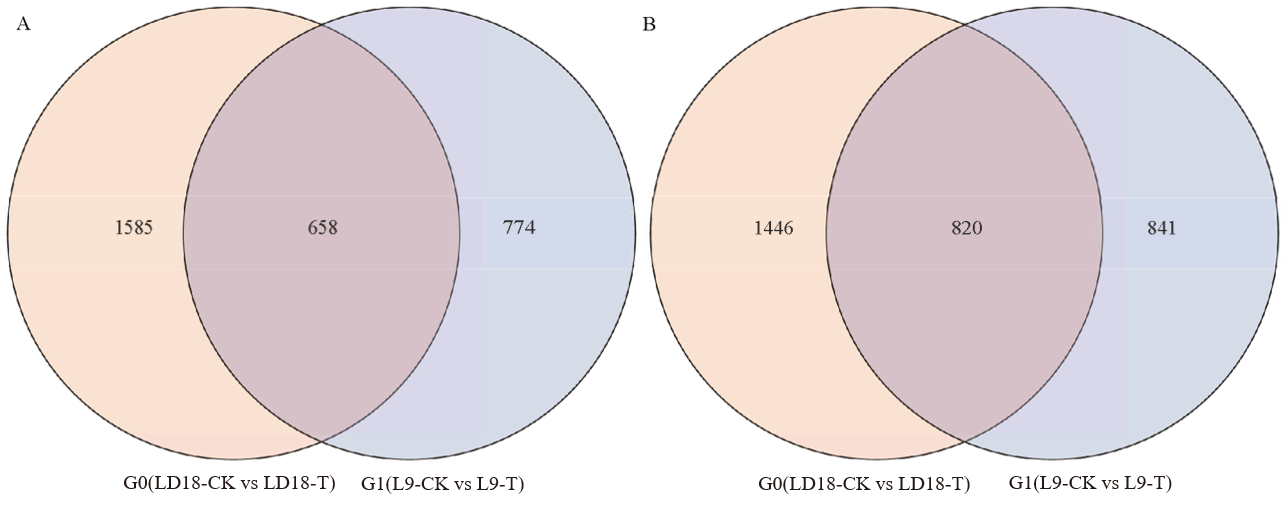
图4 孕穗期低温处理后LD18和L9均上调(A)和下调(B)表达的基因数量
Fig. 4. Up-regulated (A) and down-regulated (B) genes in LD18 and L9 compared with control at booting stage under low temperature
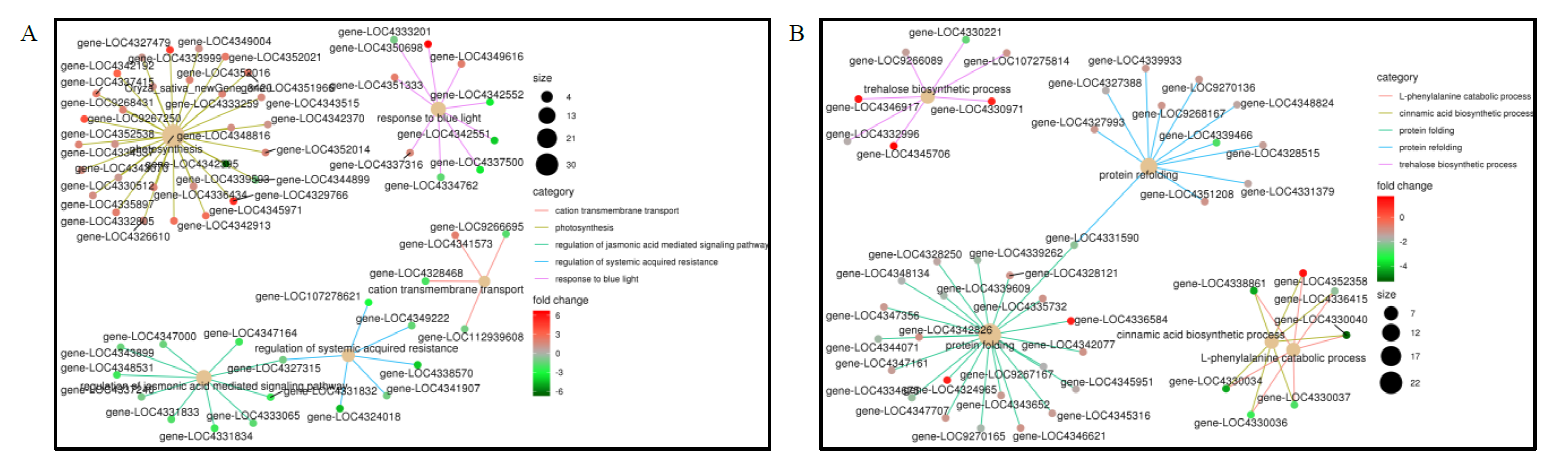
图6 孕穗期低温处理后LD18(A)和L9(B)中差异表达基因的GO功能网络
Fig. 6. GO functional network map of DEGs in LD18 and L9 under cold stress relative to CK A:LD18-CK vs LD18-T;B:L9-CK vs L9-T.
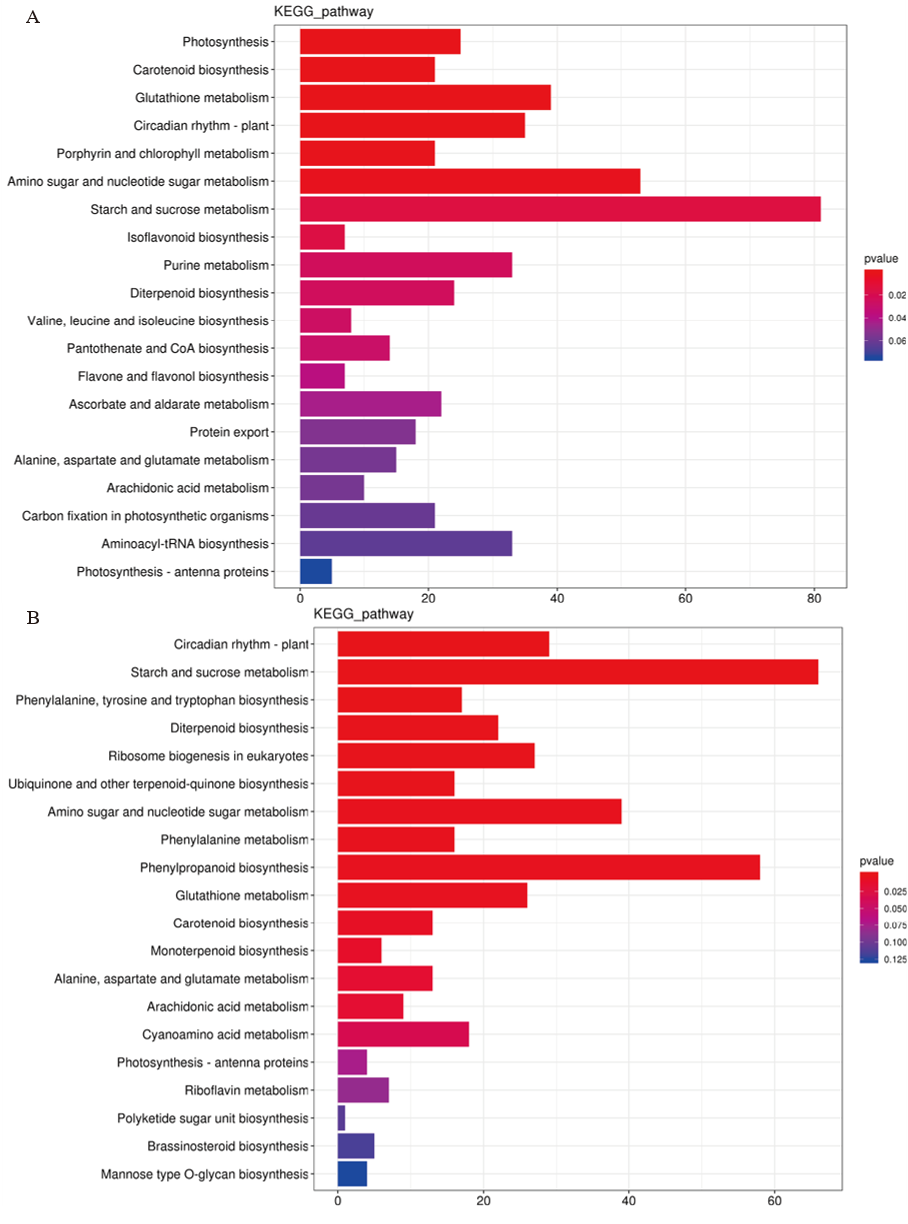
图7 孕穗期低温处理后LD18(A)和L9(B)中差异表达基因的KEGG分析
Fig. 7. KEGG analysis on DEGs in LD18 and L9 under cold stress relative to CK A:LD18-CK vs LD18-T;B:L9-CK vs L9-T.
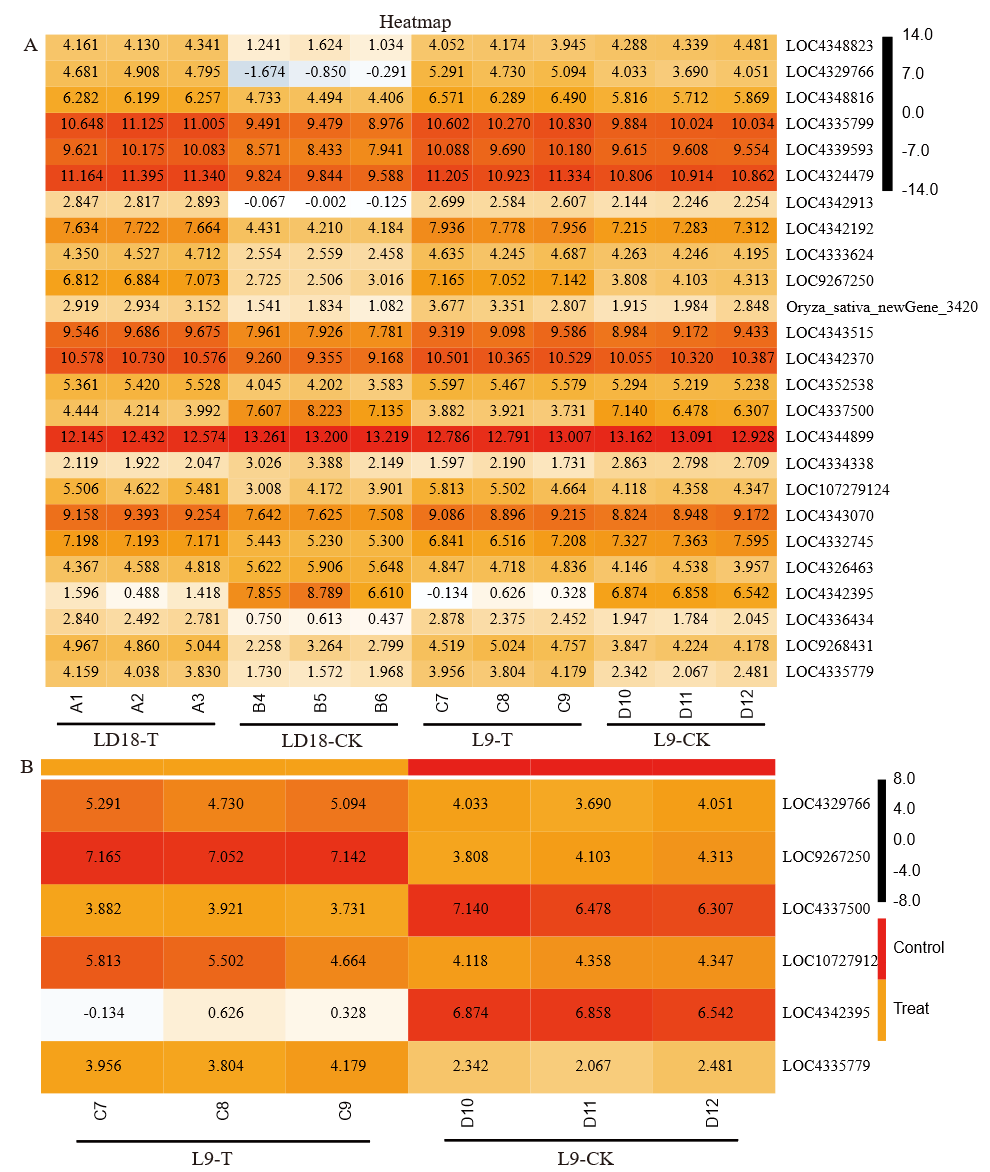
图8 LD18、L9中25个光合相关基因(A)和L9中6个光合相关差异表达基因(B)热图 每个框中数字为归一化的表达量。
Fig. 8. Heat maps of 25 photosynthesis-related genes in LD18 and L9 (A) and 6 differentially expressed photosynthesis-related genes in L9 (B) Numbers in each box are normalized expressions.
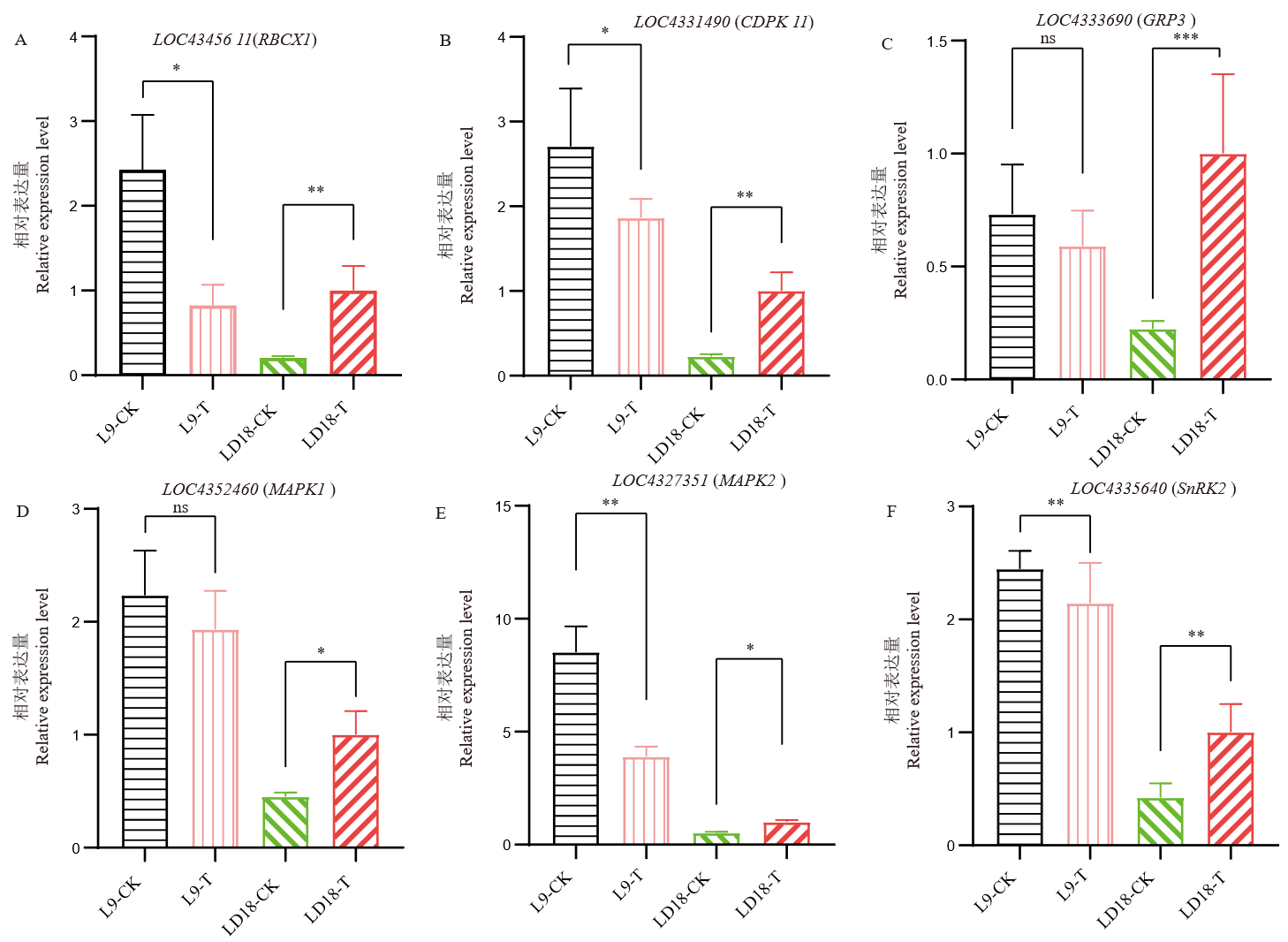
图9 孕穗期低温下,LD18和L9中部分差异基因表达分析 ns:无显著差异; *:P<0.05;**:P<0.01。
Fig. 9. RT-qPCR validation of DEGs in LD18 and L9 due to CS ns, No significant difference; *, P<0.05; **, P<0.01.
| [1] | 李文枫, 毕洪文, 黄峰华, 李晓晨, 李金霞, 张妍, 刘艳霞. 黑龙江省水稻产业发展现状及展望[J]. 农业展望, 2020, 16 (12): 48-53+64. |
| Li W F, Bi H W, Huang F H, Li X C, Li J X, Zhang Y, Liu Y X. Current Status and Prospects of Rice Industry Development in Heilongjiang Province[J]. Agricultural Outlook, 2020, 16 (12): 48-53+64. (in Chinese) | |
| [2] | 国家统计局. 中国统计年鉴[M]. 北京: 中国统计出版社, 2021. |
| National Bureau of Statistics. China Statistical Yearbook[M]. Beijing: China Statistics Press, 2021. (in Chinese) | |
| [3] | 马建勇, 许吟隆, 潘婕. 东北地区农业气象灾害的趋势变化及其对粮食产量的影响[J]. 中国农业气象, 2012, 33(2): 283-288. |
| Ma J Y, Xu Y L, Pan J. Trend changes in agricultural meteorological disasters in Northeast China and their impact on grain output[J]. Chinese Journal of Agrometeorology, 2012, 33(2): 283-288. (in Chinese with English abstract) | |
| [4] | Liu Z X, Deng H B. Development of genetic and QTLs analysis for cold tolerance in rice[J]. Chinese Agricultural Science Bulletin, 2009, 25: 45-50. |
| [5] | Erdal S. Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress[J]. Plant Physiology and Biochemistry, 2012, 57: 1-7. |
| [6] | Li J H, Zhang Z Y, Chong K, Xu Y Y. Chilling tolerance in rice: Past and present[J]. Journal of Plant Physiology, 2022, 268: 153576. |
| [7] | Li J L, Pan Y H, Guo H F, Zhou L, Yang S M, Zhang Z Y, Yang J Z, Zhang H L, Li J J, Zeng Y W, Li Z C. Fine mapping of QTL qCTB10-2 that confers cold tolerance at the booting stage in rice[J]. Theoretical and Applied Genetics, 2018, 131: 157-166. |
| [8] | 随晶晶, 赵桂龙, 金欣, 卜庆云, 唐佳琦. 水稻孕穗期耐冷调控的分子及生理机制研究进展[J/OL]. 中国水稻科学, 2025, 39(1): 1-10. |
| Sui J J, Zhao G L, Jin X, Bu Q Y, Tang J Q. Advances in molecular and physiological mechanisms of cold tolerance regulation of rice at the booting stage[J/OL]. Chinese Journal of Rice Science, 2025, 39(1): 1-10. (in Chinese with English abstract) | |
| [9] | 韦云飞, 白璐嘉, 宋晓叶, 肖晓荣, 马启林. 基于水稻幼穗盐胁迫响应转录组的MYB基因分析及耐盐基因挖掘[J]. 分子植物育种, 2023, 21(2): 360-369. |
| Wei Y F, Bai L J, Song X Y, Xiao X R, Ma Q L. Transcriptome analysis of MYB based on salt stress response in young rice panicles and mining of salt tolerance genes[J]. Molecular Plant Breeding, 2023, 21(2): 360-369. (in Chinese with English abstract) | |
| [10] | 郭震华, 马文东, 蔡丽君, 蔡永盛, 胡月婷, 韩笑, 田崇兵, 张希瑞, 王翠. 基于转录组测序的寒地水稻孕穗期低温响应分析[J/OL]. 江苏农业科学, 2024, 52(19): 34-40. |
| Guo Z H, Ma W D, Cai L J, Cai Y S, Hu Y T, Han X, Tian C B, Zhang X R, Wang C. Analysis of low temperature response during the booting stage of cold region rice based on transcriptome sequencing[J/OL]. Jiangsu Agricultural Sciences, 2024, 52(19): 34-40. (in Chinese with English abstract) | |
| [11] | 郭慧, 李树杏, 甘雨, 张宏伟, 郝留根, 杨占烈, 向关伦, 王珍珍, 易崇粉. 水稻幼苗期低温胁迫的生理响应及转录组分析[J]. 西南农业学报, 2023, 36(10): 2116-2125. |
| Guo H, Li S X, Gan Y, Zhang H W, Hao L G, Yang Z L, Xiang G L, Wang Z Z, Yi C F. Transcriptome analysis and physiological response to low temperature stress at rice seedling stage[J]. Southwest China Journal of Agricultural Sciences, 2023, 36(10): 2116-2125. (in Chinese with English abstract) | |
| [12] | 邓伟, 吕莹, 董阳均, 徐雨然, 杨华涛, 张锦文, 张建华, 奎丽梅, 涂建, 相罕章, 管俊娇, 董维, 谷安宇, 安华, 杨丽萍, 张笑, 李小林. 云南水稻种质资源的遗传多样性分析[J]. 植物遗传资源学报, 2023, 24(3): 624-635. |
| Deng W, Lü Y, Dong Y J, Xu Y R, Yang H T, Zhang J W, Zhang J H, Kui L M, Tu J, Xiang H Z, Guan J J, Dong W, Gu A Y, An H, Yang L P, Zhang X, Li X L. The genetic diversity analysis of rice germplasm resources in Yunnan Province of China[J]. Journal of Plant Genetic Resources, 2023, 24(3): 624-635. (in Chinese with English abstract) | |
| [13] | Khairy A I H, Oh M J, Lee S M, Kim D S, Roh K S. Nitric oxide overcomes Cd and Cu toxicity in in vitro-grown tobacco plants through increasing contents and activities of rubisco and rubisco activase[J]. Biochimie Open, 2016, 2: 41-51 |
| [14] | 童启庆, 须海荣. 茶叶中乙醇酸氧化酶活性测定[J]. 中国茶叶, 1990(3): 14-15. |
| Tong Q Q, Xu H R. Determination of glycolate oxidase activity in tea[J]. China Tea, 1990(3): 14-15. (in Chinese) | |
| [15] | Coyne K J, Wang Y, Wood S A, Countway P D, Greenlee S M. Current applications and technological advances in quantitative real-time PCR (qPCR): A versatile tool for the study of phytoplankton ecology[J]. Advances in Phytoplankton Ecology, 2022, 303-351. |
| [16] | Zhao J, Zhang S, Yang T, Zeng Z, Huang Z, Liu Q, Wang X, Leach J, Leung H, Liu B. Global transcriptional profiling of a cold-tolerant rice variety under moderate cold stress reveals different cold stress response mechanisms[J]. Physiologia Plantarum, 2015, 154(3): 381-394. |
| [17] | 王连敏, 王立志, 李忠杰, 李锐, 王春艳, 刘功, 中本和夫. 黑龙江省水稻品种耐寒能力评价[C]//中国作物学会栽培专业委员会换届暨学术研讨会论文集. 哈尔滨: 黑龙江省农业科学院耕作栽培所, 2007: 104-110. |
| Wang L M, Wang L Z, Li Z J, Li R, Wang C Y, Liu G. Evaluation on summer cooling injury tolerance of rice varieties in Heilongjiang Province[C]//Proceedings of the Chinese Crop Society Cultivation Professional Committee Election and Academic Seminar. Harbin: Crop Tillage and Cultivation Institute of Heilongjiang Academy of Agricultural Sciences, 2007: 104-110. | |
| [18] | Shimono H, Hasegawa T, Fujimura S, Iwama K. Responses of leaf photosynthesis and plant water status in rice to low water temperature at different growth stages[J]. Field Crops Research, 2004, 89(1): 71-83. |
| [19] | Ariizumi T, Kishitani S, Inatsugi R, Nishida I, Murata N, Toriyama K. An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings[J]. Plant & Cell Physiology, 2002, 43(7): 751-758. |
| [20] | Ben Yahmed J, de Oliveira T M, Novillo P, Quinones A, Forner M A, Salvador A, Froelicher Y, Ben Mimoun M, Talon M, Ollitrault P, Morillon R. A simple, fast and inexpensive method to assess salt stress tolerance of aerial plant part: Investigations in the mandarin group[J]. Journal of Plant Physiology, 2016, 190: 36-43. |
| [21] | Jeong S W, Choi S M, Lee D S, Ahn S N, Hur Y, Soon Chow W, Park Y I. Differential susceptibility of photosynthesis to light-chilling stress in rice (Oryza sativa L.) depends on the capacity for photochemical dissipation of light[J]. Molecules and Cells, 2002, 13(3): 419-428. |
| [22] | 蔡金桓, 薛立. 高山植物的光合生理特性研究进展[J]. 生态学杂志, 2018, 37(1): 245-254. |
| Cai J H, Xue L. Advances on photosynthesis characteristics of alpine plants[J]. Chinese Journal of Ecology, 2018, 37(1): 245-254. | |
| [23] | Mukherjee S P, Choudhuri M A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings[J]. Physiologia Plantarum, 1983, 58(2): 166-170. |
| [24] | Mittler R, Zilinskas B A. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought[J]. The Plant Journal, 1994, 5(3): 397-405. |
| [25] | Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco[J]. Plant Physiology, 2002, 130(3): 1143-1151. |
| [26] | Pant B D, Oh S, Lee H K, Nandety R S, Mysore K S. Antagonistic Regulation by CPN60A and CLPC1 of TRXL1 that regulates MDH activity leading to plant disease resistance and thermotolerance[J]. Cell Reports, 2020, 33(11): 108512. |
| [27] | Salesse-Smith C E, Sharwood R E, Busch F A, Stern D B. Increased Rubisco content in maize mitigates chilling stress and speeds recovery[J]. Plant Biotechnology Journal, 2020, 18: 1409-1420. |
| [1] | 黄福灯, 吴春艳, 郝媛媛, 韩一飞, 张小斌, 孙会锋, 潘刚. 不同氮肥水平下水稻倒二叶叶鞘的转录组分析[J]. 中国水稻科学, 2025, 39(4): 563-574. |
| [2] | 随晶晶, 赵桂龙, 金欣, 卜庆云, 唐佳琦. 水稻孕穗期耐冷调控的分子及生理机制研究进展[J]. 中国水稻科学, 2025, 39(1): 1-10. |
| [3] | 梁楚炎, 曾维, 王洁冰, 叶靖, 巫明明, 翟荣荣, 张小明, 张恒木, 叶胜海. 一个浙粳99短穗小粒突变体的鉴定及转录组分析[J]. 中国水稻科学, 2025, 39(1): 67-81. |
| [4] | 叶苗, 毛雨欣, 张德海, 康钰莹, 袁榕, 张祖建. 高光效水稻品种的叶片和冠层生理生态特征及其氮素调控机制研究进展[J]. 中国水稻科学, 2024, 38(6): 617-626. |
| [5] | 伏荣桃, 陈诚, 王剑, 赵黎宇, 陈雪娟, 卢代华. 转录组和代谢组联合分析揭示稻曲病菌的致病因子[J]. 中国水稻科学, 2024, 38(4): 375-385. |
| [6] | 伏荣桃, 王剑, 陈诚, 赵黎宇, 陈雪娟, 卢代华. 水稻幼穗响应稻曲病菌毒素胁迫早期的转录组分析[J]. 中国水稻科学, 2022, 36(5): 447-458. |
| [7] | 吴龙龙, 虞轶俊, 田仓, 张露, 黄晶, 朱练峰, 朱春权, 孔亚丽, 张均华, 曹小闯, 金千瑜. 干湿交替灌溉下施氮模式对水稻光合产物和氮转运的影响[J]. 中国水稻科学, 2022, 36(3): 295-307. |
| [8] | 崔欢, 高巧丽, 罗立新, 杨靖, 陈淳, 郭涛, 刘永柱, 黄永相, 王慧, 陈志强, 肖武名. 水稻萌发期激素信号转导和谷胱甘肽代谢转录分析[J]. 中国水稻科学, 2021, 35(6): 554-564. |
| [9] | 乔胜锋, 邓亚萍, 瞿寒冰, 张伟杨, 顾骏飞, 张耗, 刘立军, 王志琴, 杨建昌. 不同籼稻品种对低磷响应的差异及其农艺生理性状[J]. 中国水稻科学, 2021, 35(4): 396-406. |
| [10] | 刘维, 陆展华, 卢东柏, 王晓飞, 王石光, 薛皦, 何秀英. 水稻小穗簇生基因OsCL6的定位及候选基因分析[J]. 中国水稻科学, 2021, 35(3): 238-248. |
| [11] | 贾琰, 杨亮, 邹德堂, 瞿炤珺, 王敬国, 刘化龙, 王晋, 赵宏伟. 孕穗期冷水胁迫下施用外源物质对寒地粳稻氮光合效率及产量的影响[J]. 中国水稻科学, 2020, 34(5): 443-456. |
| [12] | 许赵蒙, 李利华, 高晓庆, 袁正杰, 李莘, 田旭丹, 王岚岚, 瞿绍洪. 转Pi9抗稻瘟病基因水稻株系的比较转录组分析[J]. 中国水稻科学, 2020, 34(3): 245-255. |
| [13] | 李银银, 许更文, 李俊峰, 郭佳蓉, 王志琴, 杨建昌. 水稻品种的耐低磷性及其农艺生理性状[J]. 中国水稻科学, 2018, 32(1): 51-66. |
| [14] | 施勇烽, 贺彦, 郭丹, 吕向光, 黄奇娜, 吴建利. 水稻淡绿叶突变体HM133的遗传分析与基因定位[J]. 中国水稻科学, 2016, 30(6): 603-610. |
| [15] | 赵霞1,2,#,杨华伟1,#,刘然方1,陈婷婷2,奉保华2,张彩霞2,杨雪芹2,陶龙兴2,*. 水稻热耗散对逆境的响应[J]. 中国水稻科学, 2016, 30(4): 431-440. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||