
中国水稻科学 ›› 2018, Vol. 32 ›› Issue (1): 12-22.DOI: 10.16819/j.1001-7216.2018.7055
刘小云, 李晓, 李腾飞, 苏鲁方
收稿日期:2017-05-18
修回日期:2017-09-15
出版日期:2018-01-10
发布日期:2018-01-10
基金资助:Xiaoyun LIU, Xiao LI, Tengfei LI, Lufang SU
Received:2017-05-18
Revised:2017-09-15
Online:2018-01-10
Published:2018-01-10
摘要:
【目的】 OsENO2-2是OsENO2通过可变剪切产生的短转录本,其cDNA序列从粳稻中花11中分离获得。本研究的主要目的是初步分析OsENO2-2在水稻抽穗期调控中的作用。【方法】 构建了OsENO2-2的超量表达载体,获得转基因植株,通过对表型的观察和统计,分析目的基因在过量表达条件下的功能,并利用反向遗传学方法验证该基因的功能。【结果】 OsENO2-2过量表达导致水稻在长日照条件下的抽穗期推迟,而短日照条件下抽穗期无明显变化。通过qRT-PCR方法对水稻开花关键基因的检测发现,在长日照条件下,RFT1的表达量在超表达材料中显著下调,其他开花重要基因表达量在野生型和超表达材料中没有显著变化;而在短日照条件下,所有检测基因的表达量在野生型和超表达材料中均没有显著变化。【结论】 在长日照条件下,OsENO2-2主要通过调控RFT1基因的表达来调控水稻抽穗期。
中图分类号:
刘小云, 李晓, 李腾飞, 苏鲁方. 水稻OsENO2-2基因过表达对水稻抽穗期的影响[J]. 中国水稻科学, 2018, 32(1): 12-22.
Xiaoyun LIU, Xiao LI, Tengfei LI, Lufang SU. Overexpression of OsENO2-2 Affects Heading Date in Rice[J]. Chinese Journal OF Rice Science, 2018, 32(1): 12-22.
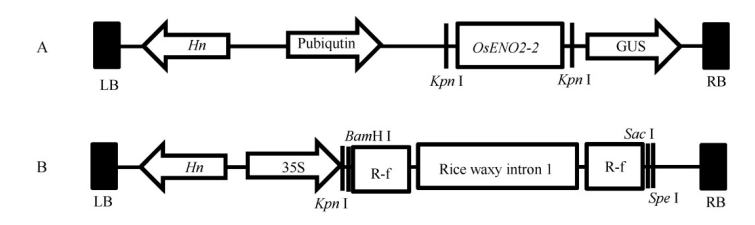
图1 超表达载体和RNAi载体的构建 A-超表达载体结构。LB为T-DNA左边界;Hn-潮霉素抗性筛选标记;Pubiqutin为玉米ubiqutin基因启动子;GUS为β-葡萄糖苷酸酶基因;RB为T-DNA右边界。B-RNAi载体结构。35S-花椰菜花叶病毒启动子;R-f为OsENO2-2 RNAi片段。
Fig. 1. Construction of overexpression vector and RNAi vector. A, Structure of overexpression vector; LB, Left border of T-DNA; Hn, Hygromycin; Pubiqutin, Promoter of ubiqutin; GUS, beta-glucuronidase; RB, Right border of T-DNA. B, Structure of RNAi vector. 35S, 35S promoter of CaMV; R-f, RNAi fragment of OsENO2-2.
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence(5′-3′) |
|---|---|
| OsENO2-2-F | GGGGGTACCATGGTTCAACAGCTTGATGGAA |
| OsENO2-2-R | GGGGGTACCTTAGTACGGCTCCACAGGGG |
| R-OsENO2-2-F | AGAACTAGTGGTACCTGCTTCCACCGGAATATATGAG |
| R-OsENO2-2-R | AGAGAGCTCGGATCCCCCTCTTTGTTTTCCTGAATGTTA |
| Hn-F | TACACAGCCATCGGTCCAGA |
| Hn-R | TAGGAGGGCGTGGATATGTC |
| OsENO2-2-QF | GGCCAAGATGCCACAAATGT |
| OsENO2-2-QR | TTGCCTGTGTAGCCAGCCTTA |
| Actin-QF | TGTATGCCAGTGGTCGTACCA |
| Actin-QR | CCAGCAAGGTCGAGACGAA |
| RFT1-QF | TGGTGTTCGTGCTGTTCCA |
| RFT1-QR | TTGTAGAGCTCGGCGAAGTTC |
| RID1-QF | CGACGACAATAGCTCGATCGC |
| RID1-QR | GTGCATGGTCACGGAGCCTT |
| Hd3a-QF | GCTCACTATCATCATCCAGCATG |
| Hd3a-QR | CCTTGCTCAGCTATTTAATTGCATAA |
| Hd1-QF | TCAG CAACAGCATATCT TTCTCATCA |
| Hd1-QR | TCTGGAATTTGGCTATACTATCACC |
| Ehd1-QF | GGATGCAAGGAAATCATGGA |
| Ehd1-QR | AATCCCATCGGAAATCTTGG |
| OsGI-QF | TGGAGAAAGGTTGTGGATGC |
| OsGI-QR | GATAGACGGCACTTCAGCAGAT |
| Ghd7-QF | AAATCCGGTACGCGTCCAG |
| Ghd7-QR | GACATAGGTGGATGGCGGTG |
表1 本研究所用引物
Table 1 Primers used in the study.
| 引物名称 Primer name | 引物序列(5′-3′) Primer sequence(5′-3′) |
|---|---|
| OsENO2-2-F | GGGGGTACCATGGTTCAACAGCTTGATGGAA |
| OsENO2-2-R | GGGGGTACCTTAGTACGGCTCCACAGGGG |
| R-OsENO2-2-F | AGAACTAGTGGTACCTGCTTCCACCGGAATATATGAG |
| R-OsENO2-2-R | AGAGAGCTCGGATCCCCCTCTTTGTTTTCCTGAATGTTA |
| Hn-F | TACACAGCCATCGGTCCAGA |
| Hn-R | TAGGAGGGCGTGGATATGTC |
| OsENO2-2-QF | GGCCAAGATGCCACAAATGT |
| OsENO2-2-QR | TTGCCTGTGTAGCCAGCCTTA |
| Actin-QF | TGTATGCCAGTGGTCGTACCA |
| Actin-QR | CCAGCAAGGTCGAGACGAA |
| RFT1-QF | TGGTGTTCGTGCTGTTCCA |
| RFT1-QR | TTGTAGAGCTCGGCGAAGTTC |
| RID1-QF | CGACGACAATAGCTCGATCGC |
| RID1-QR | GTGCATGGTCACGGAGCCTT |
| Hd3a-QF | GCTCACTATCATCATCCAGCATG |
| Hd3a-QR | CCTTGCTCAGCTATTTAATTGCATAA |
| Hd1-QF | TCAG CAACAGCATATCT TTCTCATCA |
| Hd1-QR | TCTGGAATTTGGCTATACTATCACC |
| Ehd1-QF | GGATGCAAGGAAATCATGGA |
| Ehd1-QR | AATCCCATCGGAAATCTTGG |
| OsGI-QF | TGGAGAAAGGTTGTGGATGC |
| OsGI-QR | GATAGACGGCACTTCAGCAGAT |
| Ghd7-QF | AAATCCGGTACGCGTCCAG |
| Ghd7-QR | GACATAGGTGGATGGCGGTG |
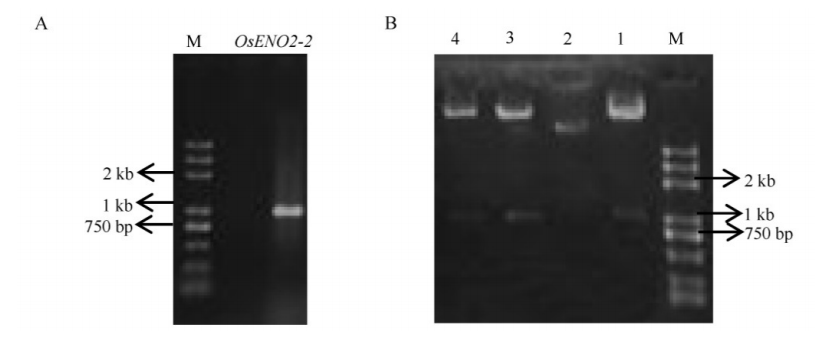
图2 OsENO2-2全长开放阅读框的PCR扩增和过表达载体的鉴定 A-OsENO2-2全长开放阅读框的PCR扩增。B-过表达载体的酶切鉴定。1~4为4个不同单克隆,其中1、3、4为阳性克隆;2-阴性对照;M为DNA标记。
Fig. 2. PCR amplification of OsENO2-2 and identification of recombinant plasmid. A, PCR amplification of OsENO2-2 full open reading frame. B, Digesting identification of recombinant plasmid. Lanes 1-4, Four independent clones of recombinant plasmid. Lane 1, 3, 4, Positive clones; Lane 2, Negative control.
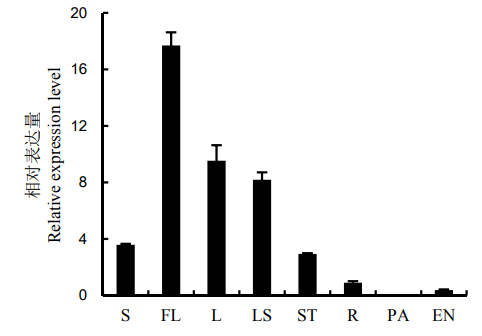
图3 OsNEO2-2组织特异性表达 S-幼苗;FL-剑叶;L-抽穗期倒2叶;LS-叶鞘;ST-茎秆;R-根;PA-幼穗;EN-胚乳。
Fig. 3. Tissue-specific expression of OsENO2-2. S, Seedling; FL, Flag leaf; L, The second leaf from the top at the heading stage; LS, Leaf sheath; ST, Stem; R, Root; PA, Panicle; EN, Endosperm.
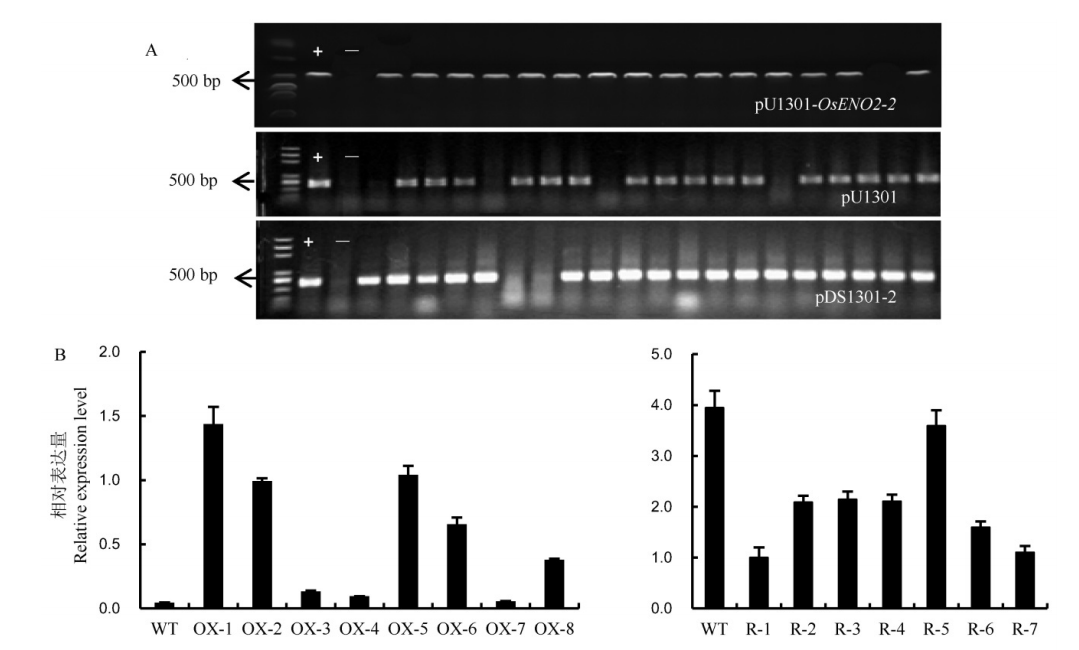
图4 T0代转基因阳性植株阳性鉴定以及表达量检测 A-转基因阳性植株Hn基因PCR鉴定,自上到下依次为超表达载体、空载体对照组、RNAi载体的转基因植株。B-转基因植株OsENO2-2的表达量(平均值±标准差)。WT-野生型;OX-1~OX-8,8个超表达转基因植株;R-1~R-7,7个RNAi转基因植株。
Fig. 4. PCR analysis of Hn gene in T0 transgenic positive plants and expression analysis of OsENO2-2 in transgenic lines. A, PCR identification of Hn gene of the transgenic plants. The transgenic plants of the overexpressed lines, the transgenic plants of the control group lines, the transgenic plants of the RNAi lines, from up to down, respectively. B, The relative expression level of OsENO2-2 in transgenic plants(Mean±SD). WT, Wild type; OX-1 to OX-8, Eight independent overexpression transgenic plants. R-1 to R-7, Seven independent RNAi transgenic plants.
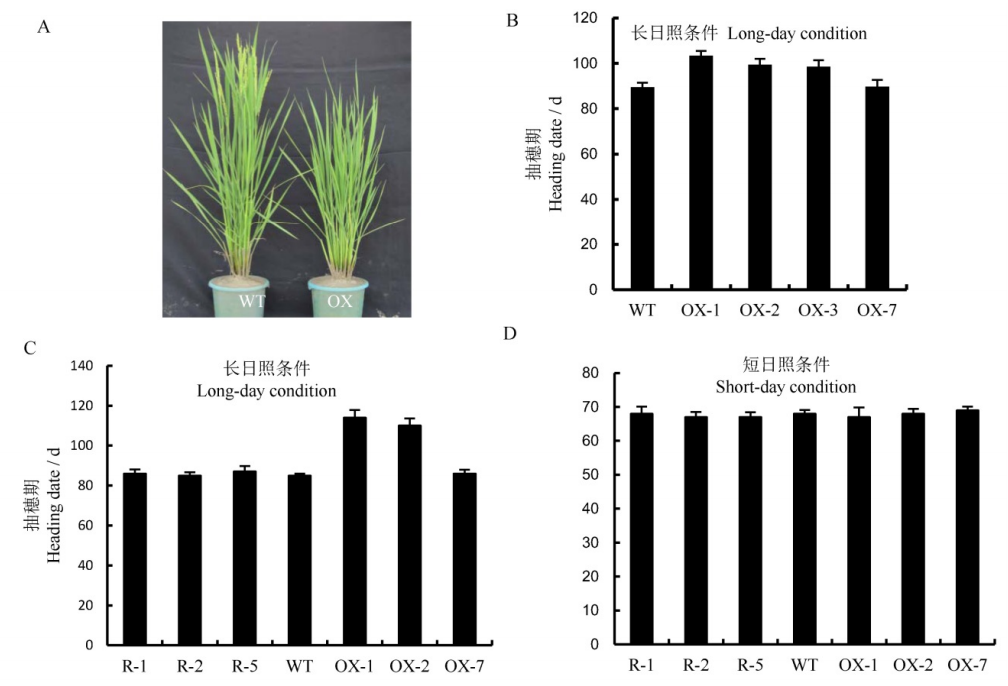
图5 转基因阳性植株的抽穗期(平均值±标准差) A-野生型和超表达植株的抽穗期表型。WT-野生型植株,OX-超表达植株; B-野生型和超表达植株在长日照大田的抽穗期(n=30)。WT-野生型植株,OX-1, OX-2, OX-3为超表达阳性株系,OX-7为转基因对照株系。LD-长日照条件。C-在长日照条件下(光照14 h/黑暗10 h)超表达,RNAi株系以及野生型株系的抽穗期(n=10)。R-1,R-2,R-5为RNAi抑制阳性株系。D-在短日照条件下(光照10 h/黑暗14 h)超表达,RNAi株系以及野生型株系的抽穗期(n=10)。SD-短日照条件。**表示与野生型植株相比差异达极显著水平(P<0.01,t检验)。
Fig. 5. Heading date of the transgenic plants(Mean±SD). A, Heading phenotypes of wild-type and overexpression plants. WT, Wild type; OX, Overexpression lines. B, Heading date of wild-type and overexpressing plants(n=30). WT, Wild type; OX-1, OX-2, OX-3, Overexpressed transgenetic positive plants; OX-7, The transgenic plants of the control group. C, Statistical analysis of heading date of wild-type, overexpressed plants and RNAi plants under long-day condition(14h light/10 h dark) (n=10). D, Statistical analysis of heading date of wild-type, overexpressed plants and RNAi plants under short-day condition(10h light/14h dark) (n=10). **The differences between wild type and transgenic plants are significant at 0.01 level(Student's t-test).
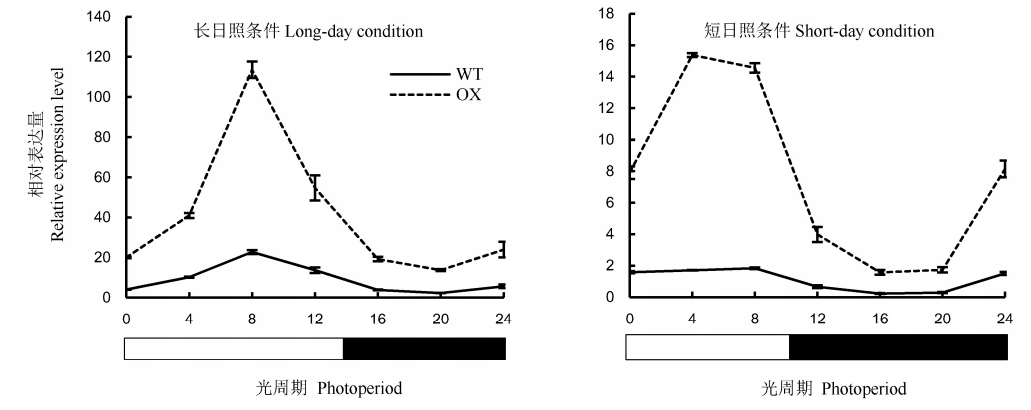
图6 OsENO2-2基因在不同光周期条件下的昼夜节律性表达(平均值±标准差) LD-长日照条件(光照14 h/黑暗10 h); SD-短日照条件(光照10 h/黑暗14 h); WT-野生型植株; OX-OsENO2-2超表达植株。
Fig. 6. Circadian rhythm expression of OsENO2-2 under different photoperiods(Mean±SD). LD, Long-day condition(light 14 h/dark 10 h); SD, Short-day condition(light 10 h/dark 14 h); WT, Wild type; OX, OsENO2-2 overexpressed plants.
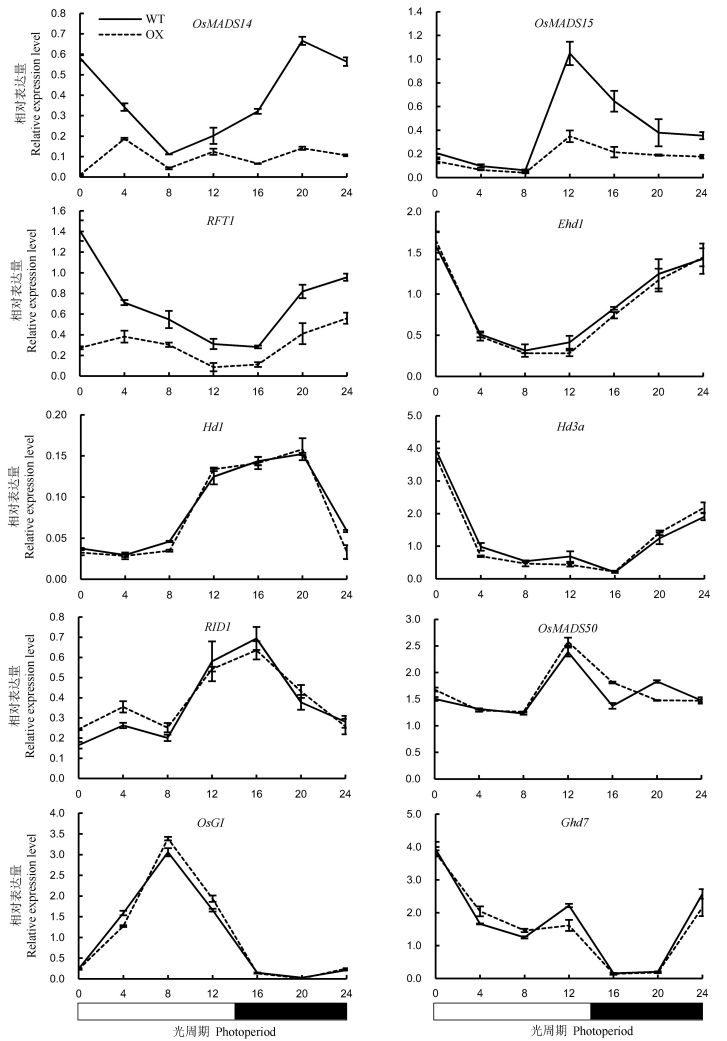
图7 长日照条件下野生型植株(WT)与OsENO2-2超表达水稻植株(OX)中抽穗期关键基因的表达量(平均值±标准差)
Fig. 7. Expression levels of key heading regulatory genes in leaves of OsENO2-2 overexpression plants(OX) compared to wild type(WT) under long-day condition(Mean±SD).
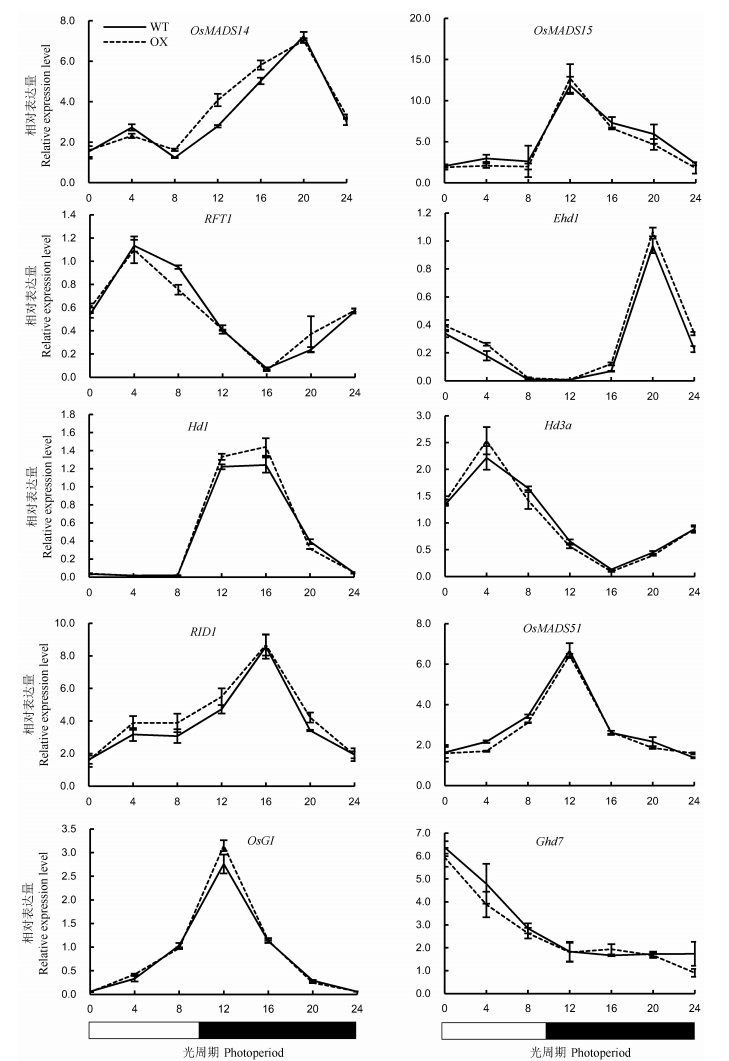
图8 短日照条件下野生型植株(WT)与OsENO2-2超表达水稻植株(OX)中抽穗期关键基因的表达量(平均值±标准差)
Fig. 8. Expression levels of key heading regulatory genes in leaves of OsENO2-2 overexpression plants(OX) compared to wild type(WT) under short-day condition(Mean±SD).
| [1] | 徐铨,奥本裕,王晓雪.水稻开花期调控分子机理研究进展.植物遗传资源学报,2014,15(1):129-136. |
| Xu Q,Okumoto Y,Wang X E.Research progress on regulatory molecular mechanisms of flowering time in riceJ Plant Genet Resour,2014,15(1):129-136. (in Chinese with English abstract) | |
| [2] | Imaizumi T,Kay S.Photoperiodic control of flowering: not only by coincidence.Trends Plant Sci,2006,11(11):550-558. |
| [3] | Itoh H,Nonoue Y,Yano M,Izawa T.A pair of floral regulators sets critical day length forHd3a florigen expression in rice. Nat Genet,2010,42(7):635-638. |
| [4] | Tsuji H,Taoka K I,Shimamoto K.Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation.Curr Opin Plant Biol,2011,14(1):45-52. |
| [5] | Yano M,Katayose Y,Ashikari M,Yamanouchi U,Monna L,Fuse T,Baba T,Yamamoto K,Umehara Y,Nagamura Y,Sasaki T. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell,2000,12(12):2473-2484. |
| [6] | Tamaki S,Matsuo S,Wong H L,Yokoi S,Shimamoto K.Hd3a protein is a mobile flowering signal in rice.Science,2007,316(5827):1033-1036. |
| [7] | Hayama R,Coupland G.The molecular basis of diversity in the photoperiodic flowering responses ofArabidopsis and rice. Plant Physiol,2004,135(2):677-684. |
| [8] | Komiya R,Yokoi S,Shimamoto K.A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice.Development,2009,136(20):3443-3450. |
| [9] | Hayama R,Yokoi S,Tamaki S,Yano M,Shimamoto K.Adaptation of photoperiodic control pathways produces short-day flowering in rice.Nature,2003,422: 719-722. |
| [10] | Kim S L,Lee S,Kim H J,Nam H G,An G.OsMADS51 is a short-day flowering promoter that functions upstream ofEhd1, OsMADS14, and Hd3a. Plant Physiol,2007,145(4):1484-1494. |
| [11] | Xue W,Xing Y,Weng X,Zhao Y,Tang W,Wang L,Zhou H,Yu S,Xu C,Li X,Zhang Q.Natural variation inGhd7 is an important regulator of heading date and yield potential in rice. Nat Genet,2008,40(6):761-767. |
| [12] | Wu C,You C,Li C,Long T,Chen G,Byrne M E,Zhang Q.RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice.Proc Natl Acad Sci USA,2008,105(35):12915-12920. |
| [13] | Matsubara K,Yamanouchi U,Wang Z X,Minobe Y,Izawa T,Yano M.Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol,2008,148(3):1425-1435. |
| [14] | Park S J,Kim S L,Lee S,Je B I,Piao H L,Park S H,Kim C M,Ryu C H,Xuan Y H,Colasanti J,An G,Han C D.RiceIndeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J,2008,56(6):1018-1029. |
| [15] | Lu S J,Wei H,Wang Y,Wang H M,Yang R F,Zhang X B,Tu J M.Overexpression of a transcription factorOsMADS15 modifies plant architecture and flowering time in rice(Oryza sativa L.). Plant Mol Biol Rep,2012,30(6):1461-1469. |
| [16] | Arora R,Agarwal P,Ray S,Singh A,Singh V,Tyagi A K,Kapoor S.MADS-box gene family in rice: Genome-wide identification, organization and expression profiling during reproductive development and stress.BMC Genom,2007,8(1):242. |
| [17] | Pancholi V.Multifunctional α-enolase: Its role in diseases.Cell Mol Life Sci,2001,58: 902-920. |
| [18] | Àngels D R,Anna R B,Ana G M,Roser L A.α-enolase, a multifunctional protein: Its role on pathophysiological situations. J Biomed Biotechnol, 2012(7):1-12. |
| [19] | Kang M,Abdelmageed H,Lee S,Reichert A,Mysore K S,Allen R D.AtMBP-1, an alternative translation product of LOS2, affects abscisic acid responses and is modulated by the E3 ubiquitin ligase AtSAP5.Plant J,2013,76(3):481-493. |
| [20] | Eremina M,Rozhon W,Yang S,Poppenberger B.ENO2 activity is required for the development and reproductive success of plants, and is feedback-repressed by AtMBP-1.Plant J,2015,81(6):895-906. |
| [21] | Sun X,Cao Y,Yang Z,Xu C,Li X,Wang S.Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J,2004,37: 517-527. |
| [22] | 卢扬江,郑康乐.提取水稻DNA 的一种简易方法.中国水稻科学,1992,6(1):47-48. |
| Lu Y J,Zheng K L.A simple method for isolation of rice DNA.Chin J Rice Sci,1992,6(1):47-48. (in Chinese with English abstract) | |
| [23] | Livak K J,Schmittgen T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] method.Methods,2001,25(4):402-408. |
| [24] | Hiei Y,Ohta S,Komari T,Kumashiro T.Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA.Plant J,1994,6(2):271-282. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||