
中国水稻科学 ›› 2016, Vol. 30 ›› Issue (2): 152-160.DOI: 10.16819/j.1001-7216.2016.5113
崔永涛, 吴立文, 胡时开, 任德勇, 葛常伟, 叶卫军, 董国军, 郭龙彪*( ), 胡兴明*(
), 胡兴明*( )
)
收稿日期:2015-07-13
修回日期:2015-10-18
出版日期:2016-03-10
发布日期:2016-03-10
通讯作者:
郭龙彪,胡兴明
基金资助:
Yong-tao CUI, Li-wen WU, Shi-kai HU, De-yong REN, Chang-wei GE, Wei-jun YE, Guo-jun DONG, Long-biao GUO*( ), Xing-ming HU*(
), Xing-ming HU*( )
)
Received:2015-07-13
Revised:2015-10-18
Online:2016-03-10
Published:2016-03-10
Contact:
Long-biao GUO, Xing-ming HU
摘要:
以粳稻品种日本晴在组织培养过程中分离出来的一个半显性矮秆突变体Si-dd1为研究对象,通过形态学分析,发现与野生型日本晴相比,矮秆Si-dd1(AA)和半矮秆Si-dd1(Aa)植株的株高降低。结实率下降,生育期延长,一次枝梗和二次枝梗增加。激素处理结果表明突变体Si-dd1(AA)与野生型对油菜素内酯反应基本相同,而在高浓度赤霉素处理下,突变体Si-dd1(AA)表现为一定程度上的钝感。Western blot对GID2表达量分析也确定这一结果。组织切片实验表明,突变体Si-dd1(AA)相对于野生型叶片主脉气孔变小,叶肉细胞增多,茎维管束数目增加。遗传分析结果表明该突变体基因受一对核基因控制。进一步利用分子标记将该基因定位在水稻第6染色体约244 kb区间内,目前该区段并未发现已报道的矮秆相关基因。
中图分类号:
崔永涛, 吴立文, 胡时开, 任德勇, 葛常伟, 叶卫军, 董国军, 郭龙彪, 胡兴明. 水稻半显性矮秆基因Si-dd1的表型分析和精细定位[J]. 中国水稻科学, 2016, 30(2): 152-160.
Yong-tao CUI, Li-wen WU, Shi-kai HU, De-yong REN, Chang-wei GE, Wei-jun YE, Guo-jun DONG, Long-biao GUO, Xing-ming HU. Phenotypical Analysis and Fine Mapping of a Semi-dominant Dwarfism Gene Si-dd1 in Rice[J]. Chinese Journal OF Rice Science, 2016, 30(2): 152-160.
| 材料 Material | 株高 Plant height /cm | 分蘖数 Tiller number | 一次枝梗 Number of primary rachis branches | 二次枝梗 Number of secondary rachis branches | 结实率 Seed-setting rate/% | 总粒数 Total grain number |
|---|---|---|---|---|---|---|
| 野生型WT | 83.8 a | 18.7 | 8.0 | 9.0 a | 88.8 a | 72.7 a |
| 半矮秆Si-dd1(Aa) | 60.6 b | 16.7 | 8.0 | 16.3 b | 61.3 b | 90.7 b |
| 矮秆Si-dd1(AA) | 43.7 c | 16.3 | 9.6 | 16.3 b | 1.1 c | 97.3 b |
表1 野生型, Si-dd1(Aa) 和Si-dd1(AA)分蘖、 株高及穗部性状比较
Table 1 Comparison of tiller, plant height and panicle traits among wild type, Si-dd1(Aa) and Si-dd1(AA).
| 材料 Material | 株高 Plant height /cm | 分蘖数 Tiller number | 一次枝梗 Number of primary rachis branches | 二次枝梗 Number of secondary rachis branches | 结实率 Seed-setting rate/% | 总粒数 Total grain number |
|---|---|---|---|---|---|---|
| 野生型WT | 83.8 a | 18.7 | 8.0 | 9.0 a | 88.8 a | 72.7 a |
| 半矮秆Si-dd1(Aa) | 60.6 b | 16.7 | 8.0 | 16.3 b | 61.3 b | 90.7 b |
| 矮秆Si-dd1(AA) | 43.7 c | 16.3 | 9.6 | 16.3 b | 1.1 c | 97.3 b |

图 1 Si-dd1突变体成熟期表型 A-整株表型; B-穗表型; C-茎节间长; D-各节间对比。从左至右依次为野生型、中间型和突变型。
Fig. 1. Phenotypes of Si-dd1 at maturation stage. A, Whole plant; B, Panicle; C, Internode; D, Comparison of every internode. From left to right,wild type, Si-dd1(Aa) and Si-dd1(AA) in turn.
| 组合 Cross | F1 | F2 | χ2(1:2:1) | |||
|---|---|---|---|---|---|---|
| 总株数 Total of plants | 正常高秆 Normal plants | 半矮秆 Semi-dwarf | 矮秆 Dwarf | |||
| Si-dd1(AA)/9311 | 半矮秆 Semi-dwarf | 184 | 48 | 94 | 42 | 0.478 |
| Si-dd1(AA)/NJ06 | 半矮秆 Semi-dwarf | 243 | 62 | 122 | 59 | 0.078 |
| NJ06/Si-dd1(AA) | 半矮秆 Semi-dwarf | 193 | 47 | 100 | 46 | 0.264 |
| 9311/Si-dd1(AA) | 半矮秆 Semi-dwarf | 290 | 72 | 150 | 68 | 0.455 |
表 2 Si-dd1(AA)与NJ06、9311组合F2的遗传分析
Table 2 Genetice analysis of F2 population of Si-dd1(AA) / NJ06(9311).
| 组合 Cross | F1 | F2 | χ2(1:2:1) | |||
|---|---|---|---|---|---|---|
| 总株数 Total of plants | 正常高秆 Normal plants | 半矮秆 Semi-dwarf | 矮秆 Dwarf | |||
| Si-dd1(AA)/9311 | 半矮秆 Semi-dwarf | 184 | 48 | 94 | 42 | 0.478 |
| Si-dd1(AA)/NJ06 | 半矮秆 Semi-dwarf | 243 | 62 | 122 | 59 | 0.078 |
| NJ06/Si-dd1(AA) | 半矮秆 Semi-dwarf | 193 | 47 | 100 | 46 | 0.264 |
| 9311/Si-dd1(AA) | 半矮秆 Semi-dwarf | 290 | 72 | 150 | 68 | 0.455 |
| 组合 Cross | 野生型 Wild type | 半矮秆 Semi-dwarf | 总株数 Total | χ2(1:1) |
|---|---|---|---|---|
| Si-dd1(Aa)/ 9311 | 54 | 48 | 102 | 0.353 |
| Si-dd1(Aa)/ NJ06 | 59 | 54 | 113 | 0.220 |
| 9311 /Si-dd1(Aa) | 52 | 45 | 97 | 0.505 |
| NJ06 /Si-dd1(Aa) | 52 | 48 | 100 | 0.160 |
表3 杂合Si-dd1(Aa)与NJ06、9311组合F1遗传分析
Table 3 Genetice analysis of F1 population of Si-dd1(Aa) /NJ06 and Si-dd1(Aa) /9311.
| 组合 Cross | 野生型 Wild type | 半矮秆 Semi-dwarf | 总株数 Total | χ2(1:1) |
|---|---|---|---|---|
| Si-dd1(Aa)/ 9311 | 54 | 48 | 102 | 0.353 |
| Si-dd1(Aa)/ NJ06 | 59 | 54 | 113 | 0.220 |
| 9311 /Si-dd1(Aa) | 52 | 45 | 97 | 0.505 |
| NJ06 /Si-dd1(Aa) | 52 | 48 | 100 | 0.160 |
| 引物名称 Primer name | 引物序列(5'-3') Forward primer(5'-3') | 引物序列(5'-3') Reverse primer(5'-3') |
|---|---|---|
| M1 | CAGTCTTGCTCCGTTTGTTG | CTGTGACTGACTTGGTCATAGG |
| M2 | ATCGCAGCAATGCCTCGTG | TGCGTTTGTGTTTGGCTCG |
| M3 | CTCAACGTTGACACCTCGTG | TCCTCCATCGAGCAGTATCA |
表4 Si-DD1基因初定位的标记
Table 4 Makers for primary mapping of Si-DD1.
| 引物名称 Primer name | 引物序列(5'-3') Forward primer(5'-3') | 引物序列(5'-3') Reverse primer(5'-3') |
|---|---|---|
| M1 | CAGTCTTGCTCCGTTTGTTG | CTGTGACTGACTTGGTCATAGG |
| M2 | ATCGCAGCAATGCCTCGTG | TGCGTTTGTGTTTGGCTCG |
| M3 | CTCAACGTTGACACCTCGTG | TCCTCCATCGAGCAGTATCA |
| 引物名称 Primer name | 引物序列(5'-3') Forward primer(5'-3') | 引物序列(5'-3') Reverse primer(5'-3') |
|---|---|---|
| P1 | GGTCGCAGCTTGAATTAATGA | GCAATCTCATTTGTTGAGAACC |
| P2 | TGATGTTTGGCACATACTTGC | GCAAACTTTCTGATAAGGAATAG |
| P3 | CTCCAAAGCTGACAATGGTG | TGAGAAGGAGTAGGAAGCATAACA |
| P4 | GGTACTAACCATGTGATTGAG | CACCTGAATTACCGTATATG |
| P5 | CGTAGGAGTCGACGCTGTC | CCCAATCCGCTGTGGTTTT |
| P6 | GCAGGTTGTAATGGAGGTGAA | CGGCGAGCCATATTGTTTAT |
表5 Si-DD1基因精细定位的部分标记
Table 5 Makers for fine mapping of Si-DD1.
| 引物名称 Primer name | 引物序列(5'-3') Forward primer(5'-3') | 引物序列(5'-3') Reverse primer(5'-3') |
|---|---|---|
| P1 | GGTCGCAGCTTGAATTAATGA | GCAATCTCATTTGTTGAGAACC |
| P2 | TGATGTTTGGCACATACTTGC | GCAAACTTTCTGATAAGGAATAG |
| P3 | CTCCAAAGCTGACAATGGTG | TGAGAAGGAGTAGGAAGCATAACA |
| P4 | GGTACTAACCATGTGATTGAG | CACCTGAATTACCGTATATG |
| P5 | CGTAGGAGTCGACGCTGTC | CCCAATCCGCTGTGGTTTT |
| P6 | GCAGGTTGTAATGGAGGTGAA | CGGCGAGCCATATTGTTTAT |
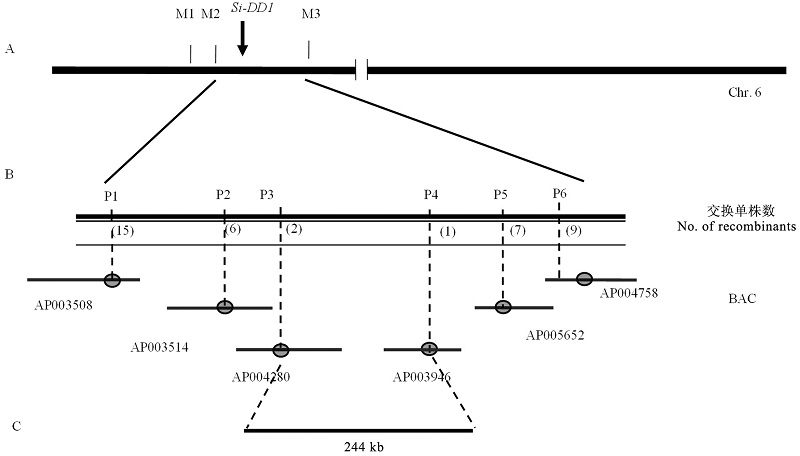
图2 Si-DD1的精细定位 A-Si-DD1与标记M2和M3连锁; B-利用4500个分离单株将Si-DD1定位到244 kb区段内。
Fig. 2. Fine mapping of Si-DD1. A,Si-DD1 was linkaged with M2 and M3; B,Si-DD1 was mapped to 244 kb genome region based on 4500 separated plants.
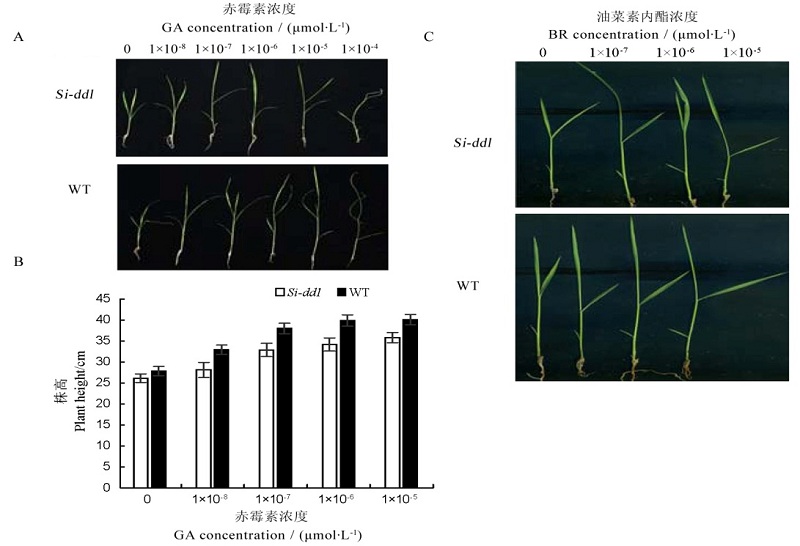
图3 Si-dd1突变体的GA, BR激素处理结果 A-不同浓度赤霉素(GA)处理后表型; B-不同浓度GA处理后株高; C-不同浓度油菜素内酯(BR)处理后叶夹角。
Fig. 3. Si-dd1 exposed to GA and BR. WT,Wild type; GA, Gibberellic acid; BR,Brassinolide. A, Plant phenotype after GA treatment at different concentrations; B, Plant height after GA treatment at different concentrations; C, Leaf angle after BR treatment at different concentrations.
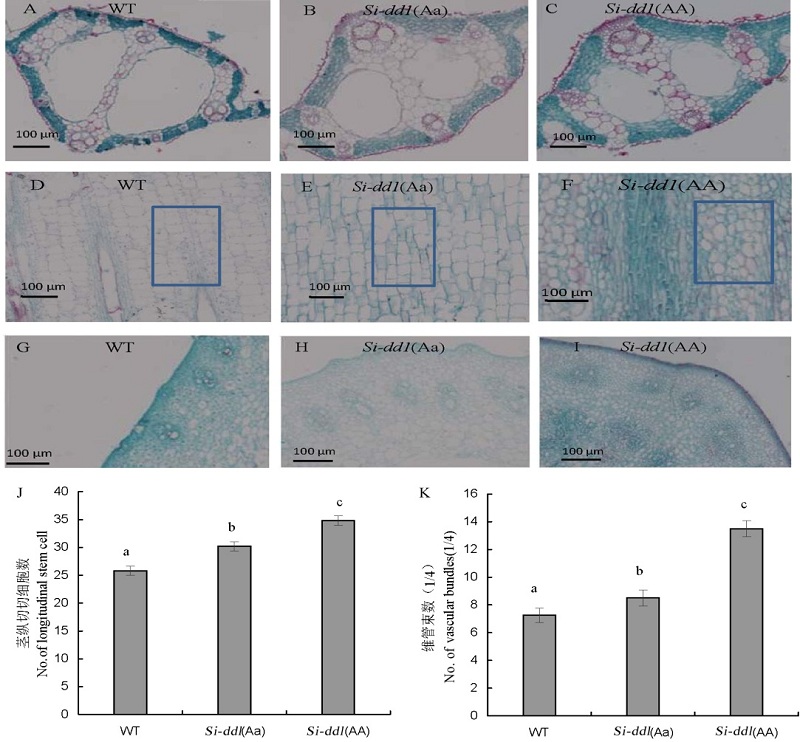
图5 野生型, Si-dd1(Aa) 和Si-dd1(AA)茎和叶的显微结构比较 A,B,C-野生型(WT), Si-dd1(Aa) 和Si-dd1(AA)叶主脉横切; D,E,F- 野生型, Si-dd1(Aa) 和Si-dd1(AA)倒2节间纵切; G,H,I- 野生型, Si-dd1(Aa) 和Si-dd1(AA)倒2节间横切; J,K-维管束(1/4)和茎纵切细胞数统计。
Fig. 5. Comparison of microscopic structure among WT, Si-dd1(Aa) and Si-dd1(AA). A,B,C, Cross sections of leaf main vein of WT, Si-dd1(Aa) and Si-dd1(AA); D,E,F, Longitudinal section of the second internode from the top of WT, Si-dd1(Aa) and Si-dd1(AA); G,H,I, Cross sections of the second internode from the top of WT, Si-dd1(Aa) and Si-dd1(AA). J,K, Numbers of vascular bundles and longitudinal stem cells.
| [1] | Spielmeyer W, Ellis M H, Chandler P M.Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene.Proc Natl Acad Sci USA, 2002, 99: 9043-9048. |
| [2] | Yin H F, Gao P, Liu C W, et al.SUI-family genes encode phosphatidylserine synthases and regulate stem development in rice.Planta, 2013, 237: 15-27. |
| [3] | Komorisono M, Ueguchi-Tanaka M, Aichi I, et al.Analysis of the rice mutant dwarf and gladius leaf 1 , aberrant katanin-mediated microtubule organization causes up-regulation of gibberellin biosynthetic genes independently of gibberellin signaling.Plant Physiol, 2005, 138: 1982-1993. |
| [4] | Iwamoto M, Kiyota S, Hanada A, et al.The multiple contributions of phytochromes to the control of internode elongation in rice.Plant Physiol, 2011, 157: 1187-1195. |
| [5] | Toyomasu T, Kagahara T, Hirose Y, et al.Cloning and characterization of cDNAs encoding ent-copalyl diphosphate synthases in wheat: Insight into the evolution of rice phytoalexin biosynthetic genes.Biosc Biotech Biochem, 2009, 73: 772-775. |
| [6] | Hirano K, Asano K, Tsuji H, et al.Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice.Plant Cell, 2010, 22: 2680-2696. |
| [7] | Ueguchi-Tanaka M, Nakajima M, Katoh E, et al.Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin.Plant Cell, 2007, 19: 2140-2155. |
| [8] | Oikawa T, Koshioka M, Kojima K, et al.A role of OsGA20ox1, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice.Plant Mol Biol, 2004, 55: 687-700. |
| [9] | Hirano K, Kouketu E, Katoh H, et al.The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity.Plant J, 2012, 71: 443-453. |
| [10] | Yang M F, Qi W W, Sun F, et al.Overexpression of rice LRK1 restricts internode elongation by down-regulating OsKO2.Biotech Lett, 2013, 35: 121-128. |
| [11] | Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, et al.Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction.Proc Natl Acad Sci USA, 2000, 97: 11638-11643. |
| [12] | Wang L, Xu Y, Ma Q, et al.Heterotrimeric G protein α subunit is involved in rice brassinosteroid response.Cell Res, 2006, 16: 916-922. |
| [13] | Hu X, Qian Q, Xu T, et al.The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G α subunit to regulate brassinosteroid-mediated growth in rice.PLoS Gene, 2013, 9: e1003391. |
| [14] | Sakamoto T, Morinaka Y, Inukai Y, et al.Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice.Plant J, 2013, 73: 676-688. |
| [15] | Hong Z, Ueguchi-Tanaka M, Umemura K, et al.A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450.Plant Cell, 2003, 15: 2900-2910. |
| [16] | Zhi H, Miyako U, Shozo F, et al.The rice brassinosteroid-deficient dwarf2 Mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone.Plant Cell, 2005, 17(8): 2243-2254. |
| [17] | Thirumurugan T, Ito Y, Kubo T, et al.Identification, characterization and interaction of HAP family genes in rice.Mol Genet Genom, 2008, 279: 279-289. |
| [18] | Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, et al.Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem.Plant J, 2002, 32: 495-508. |
| [19] | Tanabe S, Ashikari M, Fujioka S, et al.A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length.Plant Cell, 2005, 17: 776-790. |
| [20] | Bai M Y, Zhang L Y, Gampala S S, et al.Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice.Proc Natl Acad Sci USA, 2007, 104: 13839-13844. |
| [21] | Sang D J, Chen D Q, Liu G F, et al.Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis.Proc Natl Acad Sci USA, 2014, 111: 11199-11204. |
| [22] | Ito S, Kitahata N, Umehara M, et al.A new lead chemical for strigolactone biosynthesis inhibitors.Plant Cell Physiol, 2010, 51: 1143-1150. |
| [23] | Arite T, Umehara M, Ishikawa S, et al.d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers.Plant Cell Physiol, 2009, 50: 1416-1424. |
| [24] | Zou J H, Zhang S Y, Zhang W P, et al.The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds.Plant J, 2006, 48: 687-696. |
| [25] | Jiang L, Liu X, Xiong G S, et al.DWARF 53 acts as a repressor of strigolactone signalling in rice.Nature, 2014, 506: 401-405. |
| [26] | Liu W, Kohlen W, Lillo A, et al.Strigolactone biosynthesis in medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2.Plant Cell, 2011, 23: 3853-3865. |
| [27] | Guo S Y, Xu Y Y, Liu H H, et al.The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14.Nat Comm, 2013, 4(3): 1566. |
| [28] | Du H, Wu N, Fu J, et al.A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice.J Exp Bot, 2012, 63: 6467-6480. |
| [29] | Sazuka T, Kamiya N, Nishimura T, et al.A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos.Plant J, 2009, 60: 227-241. |
| [30] | Sato-Izawa K, Nakaba S, Tamura K, et al.DWARF50 (D50), a rice (Oryza sativa L.) gene encoding inositol polyphosphate 5-phosphatase, is required for proper development of intercalary meristem.Plant Cell Environ, 2012, 35: 2031-2044. |
| [31] | Zhang B C, Liu X L, Qian Q, et al.Golgi nucleotide sugar transporter modulates cell wall biosynthesis and plant growth in rice.Proc Natl Acad Sci USA, 2011, 108: 5110-5115. |
| [32] | Luan W J, Liu Y Q, Zhang F X, et al.OsCD1 encodes a putative member of the cellulose synthase-like D sub-family and is essential for rice plant architecture and growth.Plant Biotech J, 2011, 9: 513-524. |
| [33] | Rogers S O, Bendich A J.Extraction of DNA from milligram amountsof fresh, herbarium and mummified plant tissues.Plant Mol Biol, 1985, 5: 69-76. |
| [34] | Huang L M, Sun Q W, Qin F J, et al.Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice.Plant Physiol, 2007, 144: 1508-1519. |
| [35] | Zhang Q, Xu J, Li Y, et al.Morphological, anatomical and genetic analysis for a rice mutant with abnormal hull.J Genet Genom, 2007, 34: 519-526. |
| [36] | Yang D L, Li Q, Deng Y W, et al.Altered disease development in the eui mutants and Eui overexpressors indicates that gibberellins negatively regulate rice basal disease resistane.Mol Plant, 2008, 1: 528-537. |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||