
Chinese Journal OF Rice Science ›› 2025, Vol. 39 ›› Issue (3): 343-351.DOI: 10.16819/j.1001-7216.2025.240509
• Research Papers • Previous Articles Next Articles
ZHANG Bintao1,2,3, LIU Congcong1,3, GUO Mingliang3, YANG Shaohua2, WU Shiqiang5, GUO Longbiao4,*( ), ZHU Yiwang2,3,*(
), ZHU Yiwang2,3,*( )
)
Received:2024-05-16
Revised:2024-07-19
Online:2025-05-10
Published:2025-05-21
Contact:
*email: guolongbiao@caas.cn;zhuyiwang@caas.cn
张彬涛1,2,3, 刘聪聪1,3, 郭明亮3, 杨绍华2, 吴世强5, 郭龙彪4,*( ), 朱义旺2,3,*(
), 朱义旺2,3,*( )
)
通讯作者:
*email: guolongbiao@caas.cn;zhuyiwang@caas.cn
基金资助:ZHANG Bintao, LIU Congcong, GUO Mingliang, YANG Shaohua, WU Shiqiang, GUO Longbiao, ZHU Yiwang. Evaluation of Blast Resistance and Identification of Superior Haplotype of OsDR8 in Rice[J]. Chinese Journal OF Rice Science, 2025, 39(3): 343-351.
张彬涛, 刘聪聪, 郭明亮, 杨绍华, 吴世强, 郭龙彪, 朱义旺. 水稻OsDR8基因的稻瘟病抗性评价及优异单倍型鉴定[J]. 中国水稻科学, 2025, 39(3): 343-351.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2025.240509
| 引物名称 Primer name | 引物序列 Primer sequence |
|---|---|
| pEGFP-N3 | GTCGCCGTCCAGCTCGACCAG |
| Ubi-F | TTAGCCCTGCCTTCATACGC |
| OsDR8-g1-F | CAGCTTGATGGGGCTGAACCTGA |
| OsDR8-g1-R | AACTCAGGTTCAGCCCCATCAAG |
| OsDR8-g2-F | CAGCTTGACGATGAGGTCCTCGA |
| OsDR8-g2-R | AACTCGAGGACCTCATCGTCAAG |
| OsDR8-F1 | CACTCTCCTCATCCCAGAGCA |
| OsDR8-R1 | CGATCATGCCGATGTCCTGCA |
| OsDR8-F2 | TGTTACTTCTGCAGGGATCCATGGCAGCCATGGCCACC |
| OsDR8-R2 | CTCACCATAGGCCTCACGTGGGCGTCCACGATCTCGCC |
| VKOO5-F | GATGAAGTGGACGGAAGGAAGGAG |
Table 1. Primers used in this study
| 引物名称 Primer name | 引物序列 Primer sequence |
|---|---|
| pEGFP-N3 | GTCGCCGTCCAGCTCGACCAG |
| Ubi-F | TTAGCCCTGCCTTCATACGC |
| OsDR8-g1-F | CAGCTTGATGGGGCTGAACCTGA |
| OsDR8-g1-R | AACTCAGGTTCAGCCCCATCAAG |
| OsDR8-g2-F | CAGCTTGACGATGAGGTCCTCGA |
| OsDR8-g2-R | AACTCGAGGACCTCATCGTCAAG |
| OsDR8-F1 | CACTCTCCTCATCCCAGAGCA |
| OsDR8-R1 | CGATCATGCCGATGTCCTGCA |
| OsDR8-F2 | TGTTACTTCTGCAGGGATCCATGGCAGCCATGGCCACC |
| OsDR8-R2 | CTCACCATAGGCCTCACGTGGGCGTCCACGATCTCGCC |
| VKOO5-F | GATGAAGTGGACGGAAGGAAGGAG |
| 类型 Type | 元件名称 Element | 数目 Number | 功能注释 Functional annotation |
|---|---|---|---|
| 光响应元件 Light-responsive elements | G-Box | 5 | 光响应元件Light-responsive elements |
| GATA-motif | 2 | 光响应元件Light-responsive elements | |
| 激素响应元件 Hormone-responsive elements | ABRE | 4 | ABA响应元件 ABA-responsive element |
| CGTCA-motif | 3 | MeJA响应的顺式作用元件 MeJA-responsive cis-acting element | |
| TGACG-motif | 3 | MeJA响应的顺式作用元件 MeJA-responsive cis-acting element | |
| as-1 | 3 | 水杨酸响应元件 Salicylic acid-responsive element | |
| TCA | 2 | 水杨酸响应元件 Salicylic acid-responsive element | |
| MRE | 3 | 雌激素反应元件 Estrogen response element | |
| ERE | 2 | 雌激素反应元件 Estrogen response element | |
| 结合元件 Binding elements | MYB | 3 | MYB结合元件 MYB binding elements |
| MYC | 4 | MYC结合元件 MYC binding elements | |
| 胁迫相关元件 Stress-responsive elements | LTR | 2 | 低温响应元件 Low-temperature-responsive element |
| GC-motif | 2 | 防御与应激反应响应元件 Defense- and stress-responsive element | |
| TC-rich repeats | 2 | 抗病和胁迫响应元件 Disease- and stress-responsive element | |
| STRE | 5 | 应激响应元件 Stress-responsive element |
Table 2. Cis-acting elements in the promoter region of OsDR8
| 类型 Type | 元件名称 Element | 数目 Number | 功能注释 Functional annotation |
|---|---|---|---|
| 光响应元件 Light-responsive elements | G-Box | 5 | 光响应元件Light-responsive elements |
| GATA-motif | 2 | 光响应元件Light-responsive elements | |
| 激素响应元件 Hormone-responsive elements | ABRE | 4 | ABA响应元件 ABA-responsive element |
| CGTCA-motif | 3 | MeJA响应的顺式作用元件 MeJA-responsive cis-acting element | |
| TGACG-motif | 3 | MeJA响应的顺式作用元件 MeJA-responsive cis-acting element | |
| as-1 | 3 | 水杨酸响应元件 Salicylic acid-responsive element | |
| TCA | 2 | 水杨酸响应元件 Salicylic acid-responsive element | |
| MRE | 3 | 雌激素反应元件 Estrogen response element | |
| ERE | 2 | 雌激素反应元件 Estrogen response element | |
| 结合元件 Binding elements | MYB | 3 | MYB结合元件 MYB binding elements |
| MYC | 4 | MYC结合元件 MYC binding elements | |
| 胁迫相关元件 Stress-responsive elements | LTR | 2 | 低温响应元件 Low-temperature-responsive element |
| GC-motif | 2 | 防御与应激反应响应元件 Defense- and stress-responsive element | |
| TC-rich repeats | 2 | 抗病和胁迫响应元件 Disease- and stress-responsive element | |
| STRE | 5 | 应激响应元件 Stress-responsive element |
| 阳性株系 Transgenic-positive lines | 编辑率 Mutation rate(%) | 纯合型 Homozygous | 杂合型 Heterozygous | 野生型 Wild-type |
|---|---|---|---|---|
| 36 | 77.78 | 5 | 23 | 8 |
Table 3. Editing efficiency and genotypic distribution of OsDR8 through Agrobacterium-mediated genetic transformation
| 阳性株系 Transgenic-positive lines | 编辑率 Mutation rate(%) | 纯合型 Homozygous | 杂合型 Heterozygous | 野生型 Wild-type |
|---|---|---|---|---|
| 36 | 77.78 | 5 | 23 | 8 |
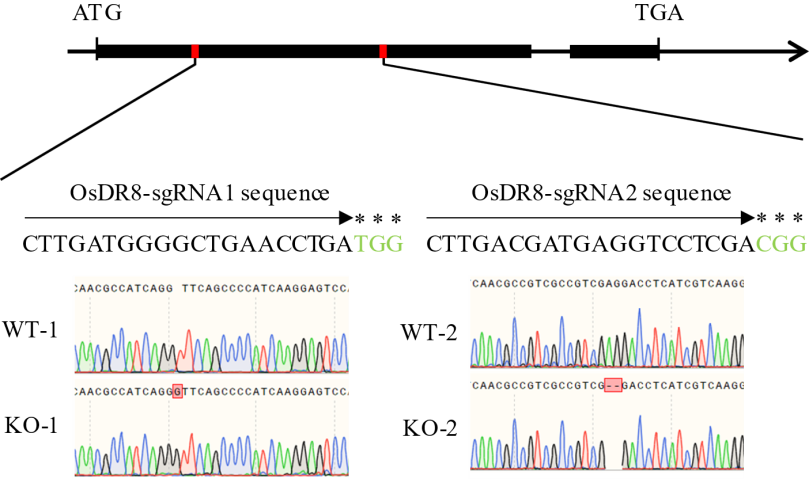
Fig. 3. Identification results of OsDR8 knockout mutants The arrows represent gRNA recognition sequences, and the asterisks denote PAM regions. WT-1 and WT-2, Wild type; KO-1 and KO-2, Knockout mutant lines.
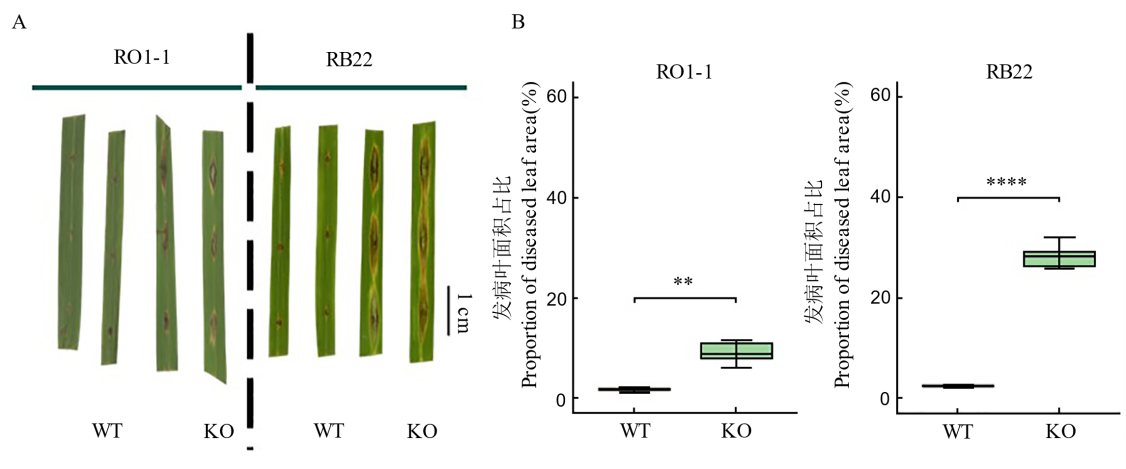
Fig. 4. Disease symptoms (A) and proportion of diseased leaf area (B) in wild-type (WT) and gene-edited materials after infection with Magnaporthe oryzae ** and **** represent significant differences between treatments at the levels of P<0.01 and P<0.0001, respectively. WT, Wild type; KO, OsDR8 knockout mutant lines. RO1-1 and RB22, Magnaporthe isolates.
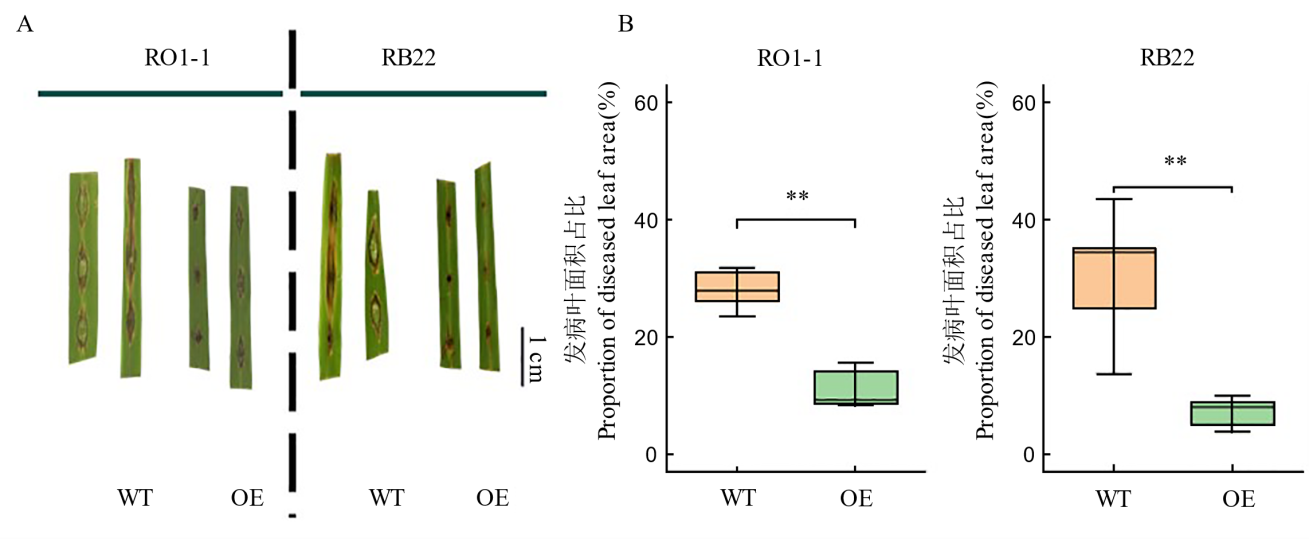
Fig. 5. Disease symptoms (A) and proportion of diseased leaf area (B) in wild-type (WT) and overexpression rice materials after infection with Magnaporthe oryzae ** represents significant differences between treatments at the level of P<0.01. WT, Wild type; OE, OsDR8 overexpression lines; RO1-1, RB22, Magnaporthe isolates.
| 单倍型Haplotype | 物理位置Physical position(bp) | 群体数量Number | ||
|---|---|---|---|---|
| 20,724,692 | 20,724,742 | 20,725,760 | ||
| Hap1 | CC | AA | AA | 58 |
| Hap2 | TT | AA | AA | 155 |
| Hap3 | CC | AA | GG | 5 |
| Hap4 | CT | AA | AA | 8 |
| Hap5 | CC | GG | AA | 4 |
Table 4. Haplotype analysis of OsDR8
| 单倍型Haplotype | 物理位置Physical position(bp) | 群体数量Number | ||
|---|---|---|---|---|
| 20,724,692 | 20,724,742 | 20,725,760 | ||
| Hap1 | CC | AA | AA | 58 |
| Hap2 | TT | AA | AA | 155 |
| Hap3 | CC | AA | GG | 5 |
| Hap4 | CT | AA | AA | 8 |
| Hap5 | CC | GG | AA | 4 |
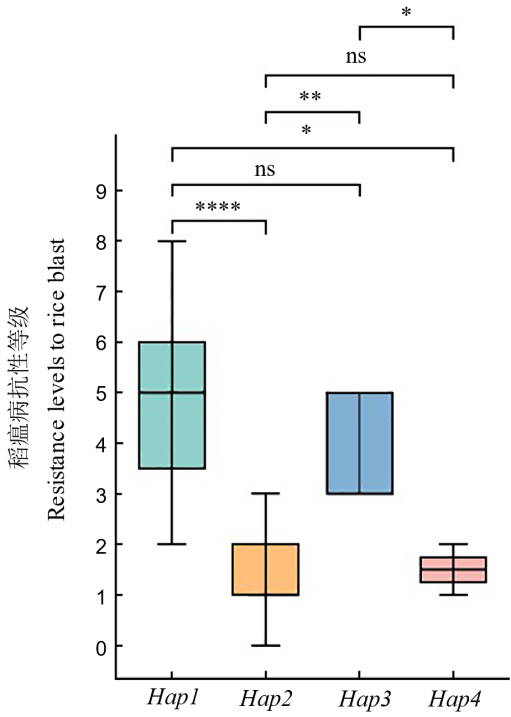
Fig. 6. Effect of haplotypes of OsDR8 on rice blast resistance ns, *, **, and **** in the figure represent non-significant difference between haplotypes and significant differences at the levels of P<0.05, P<0.01, and P<0.0001, respectively.
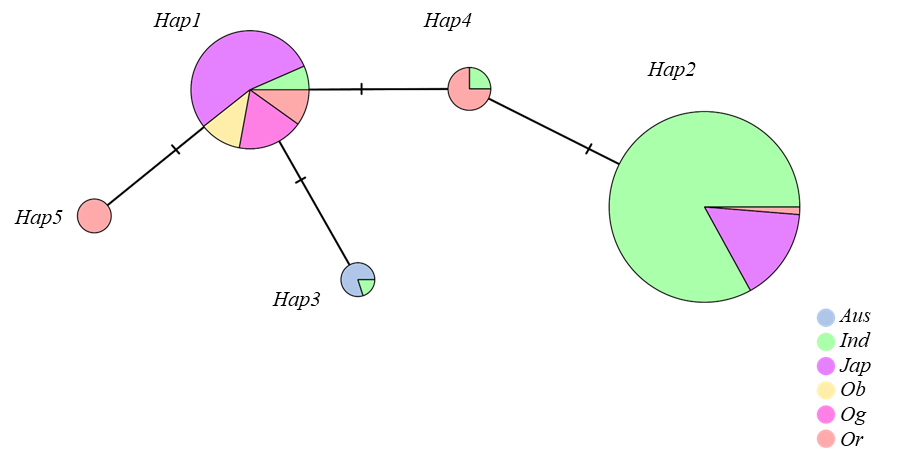
Fig. 7. Haplotype evolutionary analysis of OsDR8 In the figure, Aus, Ind, Jap, Ob, Og, and Or refer to Oryza sativa aus, O. sativa indica, O. sativa japonica, O. barthii, O. glaberrima, and O. rufipogon, respectively.
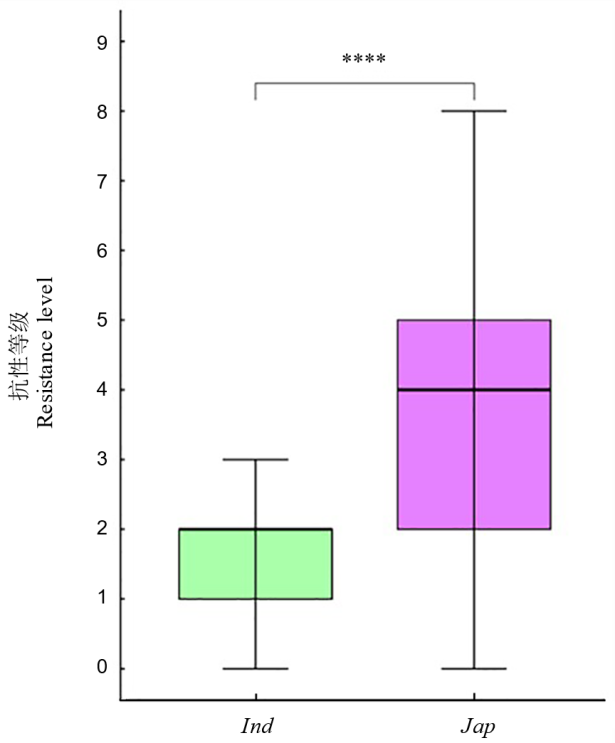
Fig. 9. Differences in resistance levels to rice blast among different subgroups of Asian cultivated rice **** in the figure represents significant differences between subgroups at the level of P<0.0001.
| [1] | Skamnioti P, Gurr S J. Against the grain: Safeguarding rice from rice blast disease[J]. Trends in Biotechnology, 2009, 27(3): 141-150. |
| [2] | Jones J D G, Dangl J L. The plant immune system[J]. Nature, 2006, 444(7117): 323-329. |
| [3] | Zhou J M, Zhang Y. Plant immunity: Danger perception and signaling[J]. Cell, 2020, 181(5): 978-989. |
| [4] | Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou J M, He S Y, Xin X F. Pattern-recognition receptors are required for NLR-mediated plant immunity[J]. Nature, 2021, 592(7852): 105-109. |
| [5] | Ngou B P M, Ahn H K, Ding P, Jones J D G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors[J]. Nature, 2021, 592(7852): 110-115. |
| [6] | Wang J, Song W, Chai J. Structure, biochemical function, and signaling mechanism of plant NLRs[J]. Molecular Plant, 2023, 16(1): 75-95. |
| [7] | Ding L N, Li Y T, Wu Y Z, Li T, Geng R, Cao J, Zhang W, Tan X L. Plant disease resistance-related signaling pathways: Recent progress and future prospects[J]. International Journal of Molecular Sciences, 2022, 23(24): 16200. |
| [8] | Dong W, Stockwell V O, Goyer A. Enhancement of thiamin content in Arabidopsis thaliana by metabolic engineering[J]. Plant and Cell Physiology, 2015, 56(12): 2285-2296. |
| [9] | Boubakri H, Gargouri M, Mliki A, Brini F, Chong J, Jbara M J P. Vitamins for enhancing plant resistance[J]. Planta, 2016, 244(3): 529-543. |
| [10] | Rapala-Kozik M, Wolak N, Kujda M, Banas A K. The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response[J]. BMC Plant Biology, 2012, 12(1): 2. |
| [11] | Ahn I P, Kim S, Lee Y H. Vitamin B1 functions as an activator of plant disease resistance[J]. Plant Physiology, 2005, 138(3): 1505-1515. |
| [12] | Ahn I P, Kim S, Lee Y H, Suh S C. Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis[J]. Plant Physiology, 2007, 143(2): 838-848. |
| [13] | Bahuguna R N, Joshi R, Shukla A, Pandey M, Kumar J. Thiamine primed defense provides reliable alternative to systemic fungicide carbendazim against sheath blight disease in rice (Oryza sativa L.)[J]. Plant Physiology and Biochemistry, 2012, 57: 159-167. |
| [14] | Wang G, Ding X, Yuan M, Qiu D, Li X, Xu C, Wang S. Dual function of rice OsDR8 gene in disease resistance and thiamine accumulation[J]. Plant Molecular Biology, 2006, 60(3): 437-449. |
| [15] | Xie X, Ma X, Zhu Q, Zeng D, Li G, Liu Y G. CRISPR-GE: A convenient software toolkit for CRISPR-based genome editing[J]. Molecular Plant, 2017, 10(9): 1246-1249. |
| [16] | Shang L, He W, Wang T, Yang Y, Xu Q, Zhao X, Yang L, Zhang H, Li X, Lü Y, Chen W, Cao S, Wang X, Zhang B, Liu X, Yu X, He H, Wei H, Leng Y, Shi C, Guo M, Zhang Z, Zhang B, Yuan Q, Qian H, Cao X, Cui Y, Zhang Q, Dai X, Liu C, Guo L, Zhou Y, Zheng X, Ruan J, Cheng Z, Pan W, Qian Q. A complete assembly of the rice Nipponbare reference genome[J]. Molecular Plant, 2023, 16(8): 1232-1236. |
| [17] | Shang L, Li X, He H, Yuan Q, Song Y, Wei Z, Lin H, Hu M, Zhao F, Zhang C, Li Y, Gao H, Wang T, Liu X, Zhang H, Zhang Y, Cao S, Yu X, Zhang B, Zhang Y, Tan Y, Qin M, Ai C, Yang Y, Zhang B, Hu Z, Wang H, Lü Y, Wang Y, Ma J, Wang Q, Lu H, Wu Z, Liu S, Sun Z, Zhang H, Guo L, Li Z, Zhou Y, Li J, Zhu Z, Xiong G, Ruan J, Qian Q. A super pan-genomic landscape of rice[J]. Cell Research, 2022, 32(10): 878-896. |
| [18] | Cingolani P, Platts A, Wang L L, Coon M, Nguyen T, Wang L, Land S J, Lu X, Ruden D M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila Melanogaster strain w1118; iso-2; iso-3[J]. Fly, 2012, 6(2): 80-92. |
| [19] | Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models[J]. Biometrical Journal, 2008, 50(3): 346-363. |
| [20] | Paradis E. PEGAS: An R package for population genetics with an integrated-modular approach[J]. Bioinformatics, 2010, 26(3): 419-420. |
| [21] | Leigh J W, Bryant D. PopART: Full-feature software for haplotype network construction[J]. Methods in Ecology and Evolution, 2015, 6(9): 1110-1116. |
| [22] | Katoh K, Standley D M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability[J]. Molecular Biology and Evolution, 2013, 30(4): 772-780. |
| [23] | Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11[J]. Molecular Biology and Evolution, 2021, 38(7): 3022-3027. |
| [24] | Ogata Y, Kimura N, Sano R. Gcorn plant: A database for retrieving functional and evolutionary traits of plant genes[J]. Plant Physiology, 2019, 180(2): 732-742. |
| [25] | Chow C N, Lee T Y, Hung Y C, Li G Z, Tseng K C, Liu Y H, Kuo P L, Zheng H Q, Chang W C. PlantPAN3.0: A new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants[J]. Nucleic Acids Research, 2019, 47(D1): D1115-D1163. |
| [26] | Liu M H, Kang H, Xu Y, Peng Y, Wang D, Gao L, Wang X, Ning Y, Wu J, Liu W, Li C, Liu B, Wang G L. Genome-wide association study identifies an NLR gene that confers partial resistance to Magnaporthe oryzae in rice[J]. Plant Biotechnology Journal, 2020, 18(6): 1376-1383. |
| [1] |
TANG Chenghan, CHEN Huizhe, HUAI Yan, SUN Liang, ZHANG Yuping, XIANG Jing, ZHANG Yikai, WANG Zhigang, XU Yiwen, WANG Yaliang.
Response of Machine-transplanting Quality and Yield Formation of Hybrid Rice Pot-mat Seedlings to Pot Depth [J]. Chinese Journal OF Rice Science, 2025, 39(4): 491-500. |
| [2] |
ZHU Peng, LING Xitie, WANG Jinyan, ZHANG Baolong, YANG Yuwen, XU Ke, QIU Shi.
Effects of Weed Control Methods on Grain Yield and Quality of Herbicide-resistant Rice Under Direct Seeding [J]. Chinese Journal OF Rice Science, 2025, 39(4): 501-515. |
| [3] |
DONG Liqiang, ZHANG Yikai, YANG Tiexin, FENG Yingying, MA Liang, LIANG Xiao, ZHANG Yuping, LI Yuedong.
Effect of Dense Sowing Nursery on Seedling Quality and Picking Characteristics for Mechanized Transplanting inNorthern japonica Rice [J]. Chinese Journal OF Rice Science, 2025, 39(4): 516-528. |
| [4] |
ZHOU Yang, YE Fan, LIU Lijun.
Research Progress of Typical Plant Growth-promoting Microorganism Enhancing Salt Stress Resistance in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(4): 529-542. |
| [5] |
ZHU Jianping, LI Xia, LI Wenqi, XU Yang, WANG Fangquan, TAO Yajun, JIANG Yanjie, CHEN Zhihui, FAN Fangjun, YANG Jie, YANG Jie.
Phenotypic Analysis and Gene Mapping of a Floury Endosperm Mutant we1 in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(4): 543-551. |
| [6] | HUANG Fudeng, WU Chunyan, HAO Yuanyuan, HAN Yifei, ZHANG Xiaobin, SUN Huifeng, PAN Gang . Transcriptome Analysis of Top Second Leaf Sheath of Rice Under Different Nitrogen Fertilizer Levels [J]. Chinese Journal OF Rice Science, 2025, 39(4): 563-571. |
| [7] | LU Yezi, QIU Jiehua, JIANG Nan, KOU Yanjun, SHI Huanbin. Research Progress in Effectors of Magnaporthe oryzae [J]. Chinese Journal OF Rice Science, 2025, 39(3): 287-294. |
| [8] | WANG Chaorui, ZHOU Yukun, WEN Ya, ZHANG Ying, FA Xiaotong, XIAO Zhilin, ZHANG Hao. Effects of Straw Returning Methods on Soil Characteristics and Greenhouse Gas Emissions in Paddy Fields and Their Regulation Through Water-fertilizer Interactions [J]. Chinese Journal OF Rice Science, 2025, 39(3): 295-305. |
| [9] | WANG Yaxuan, WANG Xinfeng, YANG Houhong, LIU Fang, XIAO Jing, CAI Yubiao, WEI Qi, FU Qiang, WAN Pinjun. Recent Advances in Mechanisms of Adaptation of Planthoppers to Rice Resistance [J]. Chinese Journal OF Rice Science, 2025, 39(3): 306-321. |
| [10] | HUANG Tao, WEI Zhaogen, CHENG Qi, CHENG Ze, LIU Xin, WANG Guangda, HU Keming, XIE Wenya, CHEN Zongxiang, FENG Zhiming, ZUO Shimin. Gene Cloning and Broad-spectrum Disease Resistance Analysis of Rice Lesion Mimic Mutant lm52 [J]. Chinese Journal OF Rice Science, 2025, 39(3): 322-330. |
| [11] | MA Shunting, HU Yungao, GAO Fangyuan, LIU Liping, MOU Changling, LÜ Jianqun, SU Xiangwen, LIU Song, LIANG Yuyu, REN Guangjun, GUO Hongming. Functional Study of Rice Eukaryotic Translation Initiation Factor OseIF6.2 in Grain Size Regulation [J]. Chinese Journal OF Rice Science, 2025, 39(3): 331-342. |
| [12] | WEI Xinyu, ZENG Yuehui, XIAO Changchun, HUANG Jianhong, RUAN Hongchun, YANG Wangxing, ZOU Wenguang, XU Xuming. Cloning and Functional Verification of Rice-Blast Resistance Gene Pi-kf2(t) in Kangfeng B [J]. Chinese Journal OF Rice Science, 2025, 39(3): 352-364. |
| [13] | LI Wenqi, XU Yang, WANG Fangquan, ZHU Jianping, TAO Yajun, LI Xia, FAN Fangjun, JIANG Yanjie, CHEN Zhihui, YANG Jie. Development and Application of KASP Marker for Broad-Spectrum Resistance Gene PigmR to Rice Blast [J]. Chinese Journal OF Rice Science, 2025, 39(3): 365-372. |
| [14] | WEI Huanhe, WANG Lulu, MA Weiyi, ZHANG Xiang, ZUO Boyuan, GENG Xiaoyu, ZHU Wang, ZHU Jizou, MENG Tianyao, CHEN Yinglong, GAO Pinglei, XU Ke, DAI Qigen. Grain-filling Characteristics and Its Relationship with Grain Yield Formation of japonica Rice Nanjing 9108 Under Combined Salinity-drought Stress [J]. Chinese Journal OF Rice Science, 2025, 39(3): 373-386. |
| [15] | ZHANG Haiwei, GU Xinyi, CHEN Mingshuai, LI Fukang, SHI Yuecheng, YANG Ting, JIANG Shuochen. Effects of Nitrogen Type of Basal Fertilizer on Growth, Grain Yield and Nitrogen Use Efficiency of Ratooning Rice [J]. Chinese Journal OF Rice Science, 2025, 39(3): 387-398. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||