
中国水稻科学 ›› 2016, Vol. 30 ›› Issue (3): 232-238.DOI: 10.16819/j.1001-7216.2016.5156
叶卫军1,2, 胡时开2,3, 吴立文2, 郭龙彪2, 钱前2,3,*( )
)
收稿日期:2015-10-26
修回日期:2015-12-19
出版日期:2016-05-10
发布日期:2016-05-10
通讯作者:
钱前
作者简介:# 共同第一作者;
基金资助:
Wei-jun YE1,2, Shi-kai HU2,3, Li-wen WU2, Long-biao GUO2, Qian QIAN2,3,*( )
)
Received:2015-10-26
Revised:2015-12-19
Online:2016-05-10
Published:2016-05-10
Contact:
Qian QIAN
About author:# These authors contributed equally to this work;
摘要:
在EMS诱变的93-11突变体库中筛选到一个稳定遗传的迟抽穗突变体dth9 (days to heading 9)。该突变体的抽穗期比野生型延长了50d左右,其他农艺性状基本无异。遗传分析表明迟抽穗性状受一个隐性核基因控制。以突变体dth9与日本晴和武运粳7号杂交构建的F2分离群体作为定位群体,利用SSR标记和新开发的8个InDel标记,将DTH9定位在第9染色体着丝粒附近D9-9和D9-17之间240 kb的区间内,该区域尚未发现与抽穗期有关的基因。此外,实时荧光定量PCR结果表明,在突变体dth9中与抽穗期相关基因的表达量显著降低。
中图分类号:
叶卫军, 胡时开, 吴立文, 郭龙彪, 钱前. 水稻迟抽穗突变体dth9的遗传分析与基因定位[J]. 中国水稻科学, 2016, 30(3): 232-238.
Wei-jun YE, Shi-kai HU, Li-wen WU, Long-biao GUO, Qian QIAN. Genetic Analysis and Gene Mapping of a Heading-delayed Mutant dth9 in Rice (Oryza sativa L.)[J]. Chinese Journal OF Rice Science, 2016, 30(3): 232-238.
| 分子标记 Marker | 正向引物 (5'-3') Forward primer (5'-3') | 反向引物 (5'-3') Reverse primer (5'-3') | 大小 Size/bp | 物理位置 Physical position/Mb |
|---|---|---|---|---|
| D9-4 | AGCCTCATACCTCCCACA | CGCCTGGAAGACAATCAA | 151 | 3.047 |
| D9-7 | AAAGATTCTCAAGGCCAGTC | TATCTAGATCGTGGCCCA | 172 | 3.583 |
| D9-8 | TTGCATGGTCACGTTCCT | TGATTGCGGAGTGATGAG | 260 | 3.608 |
| D9-9 | CCAATGTAGCAGCCGTAA | CGTTGAGGATTCAGTGGT | 129 | 3.983 |
| D9-17 | AATCGGTGAATGTCCTTG | GAAACATCCATGCCTTGC | 124 | 4.223 |
| D9-19 | TCCATCGCATTTGAGTGT | AAGTTAGTAGGCGGAAGG | 223 | 4.332 |
| D9-12 | GGGGTGATGCTGGTTTAT | AAGGGTCTCATCTGGAAAA | 255 | 4.354 |
| D9-2 | GGCTTCTCAACCAAGGTAA | ACGCATCAAATCAGGCAC | 205 | 4.554 |
| RM444 | GCTCCACCTGCTTAAGCATC | TGAAGACCATGTTCTGCAGG | 162 | 5.925 |
表1 本研究中精细定位所用引物
Table 1 Primers used for fine mapping in the study.
| 分子标记 Marker | 正向引物 (5'-3') Forward primer (5'-3') | 反向引物 (5'-3') Reverse primer (5'-3') | 大小 Size/bp | 物理位置 Physical position/Mb |
|---|---|---|---|---|
| D9-4 | AGCCTCATACCTCCCACA | CGCCTGGAAGACAATCAA | 151 | 3.047 |
| D9-7 | AAAGATTCTCAAGGCCAGTC | TATCTAGATCGTGGCCCA | 172 | 3.583 |
| D9-8 | TTGCATGGTCACGTTCCT | TGATTGCGGAGTGATGAG | 260 | 3.608 |
| D9-9 | CCAATGTAGCAGCCGTAA | CGTTGAGGATTCAGTGGT | 129 | 3.983 |
| D9-17 | AATCGGTGAATGTCCTTG | GAAACATCCATGCCTTGC | 124 | 4.223 |
| D9-19 | TCCATCGCATTTGAGTGT | AAGTTAGTAGGCGGAAGG | 223 | 4.332 |
| D9-12 | GGGGTGATGCTGGTTTAT | AAGGGTCTCATCTGGAAAA | 255 | 4.354 |
| D9-2 | GGCTTCTCAACCAAGGTAA | ACGCATCAAATCAGGCAC | 205 | 4.554 |
| RM444 | GCTCCACCTGCTTAAGCATC | TGAAGACCATGTTCTGCAGG | 162 | 5.925 |
| 分子标记 Marker | 正向引物 (5'-3') Forward primer (5'-3') | 反向引物 (5'-3') Reverse primer (5'-3') |
|---|---|---|
| Ghd7 | AGGTGCTACGAGAAGCAAATCC | GGGCCTCATCTCGGCATAG |
| Ghd8 | CGTGCAATGGTTTAGACTAAAG | AACAGCATCAGCATCAACAA |
| Hd6 | ACGTGAAGCTATGGCACATC | TGTGGTCGTGCTCTGCTATT |
| Hd3a | GCTAACGATGATCCCGAT | CCTGCAATGTATAGCATGC |
| Hd1 | CGTTTCGCCAAGAGATCAG | AGATAGAGCTGCAGTGGAGAAC |
| RFT1 | CGTCCATGGTGACCCAACA | CCGGGTCTACCATCACGAGT |
| Ehd1 | AATCGATTCCAACAACAAGCAA | TGTCGAGAGCGGTGGATGA |
| OsMADS51 | GTCGGCAAGCTCTACGAGTACTC | GCGAATTGCTGATAGCGATCA |
| OsActin1 | GCTATGTACGTCGCCATCCA | GGACAGTGTGGCTGACACCAT |
表2 抽穗期相关基因表达量分析引物
Table 2 Primers used for qRT-PCR analysis of genes associated with heading date.
| 分子标记 Marker | 正向引物 (5'-3') Forward primer (5'-3') | 反向引物 (5'-3') Reverse primer (5'-3') |
|---|---|---|
| Ghd7 | AGGTGCTACGAGAAGCAAATCC | GGGCCTCATCTCGGCATAG |
| Ghd8 | CGTGCAATGGTTTAGACTAAAG | AACAGCATCAGCATCAACAA |
| Hd6 | ACGTGAAGCTATGGCACATC | TGTGGTCGTGCTCTGCTATT |
| Hd3a | GCTAACGATGATCCCGAT | CCTGCAATGTATAGCATGC |
| Hd1 | CGTTTCGCCAAGAGATCAG | AGATAGAGCTGCAGTGGAGAAC |
| RFT1 | CGTCCATGGTGACCCAACA | CCGGGTCTACCATCACGAGT |
| Ehd1 | AATCGATTCCAACAACAAGCAA | TGTCGAGAGCGGTGGATGA |
| OsMADS51 | GTCGGCAAGCTCTACGAGTACTC | GCGAATTGCTGATAGCGATCA |
| OsActin1 | GCTATGTACGTCGCCATCCA | GGACAGTGTGGCTGACACCAT |

图1 野生型和迟抽穗突变体dth9的表型 A-93-11和突变体的植株表型,标尺=20 cm。B-DTH9成熟期表型,标尺=20 cm。
Fig. 1. Phenotypes of the wild-type and the heading-delayed mutant dth9. A, Phenotypes of the wild-type and the heading-delayed mutant DTH9, bar=20 cm. B, Phenotype of DTH9 at mature stage, bar=20 cm.
| 农艺性状 Agronomic trait | 野生型 Wild-type | 突变体 Mutant |
|---|---|---|
| 株高Plant height /cm | 118.67±1.52 | 115.30±0.58* |
| 穗长Panicle length/cm | 23.05±0.91 | 21.33±1.15 |
| 有效穗数 No. of effective panicles | 8.3±0.5 | 8.0±0.8 |
| 抽穗期 Heading date/d | 98.0±0.8 | 147.3±2.5** |
| 每穗实粒数 No. of filled grains per panicle | 171.5±4.0 | 173.0±2.7 |
| 结实率 Seed-setting rate/% | 93.72±0.01 | 92.20±0.01 |
| 千粒重 1000-grain weight/g | 31.50±0.57 | 31.57±0.40 |
| 一次枝梗数 Primary rachis branch number | 12.0±0.7 | 12.6±0.6 |
| 二次枝梗数 Secondary rachis branch number | 36.8±1.3 | 37.3±0.6 |
表3 野生型和突变体的主要农艺性状比较(浙江杭州,2013年)
Table 3 Comparison of major agronomic traits between the wild-type and the mutant (Hangzhou, Zhejiang, 2013).
| 农艺性状 Agronomic trait | 野生型 Wild-type | 突变体 Mutant |
|---|---|---|
| 株高Plant height /cm | 118.67±1.52 | 115.30±0.58* |
| 穗长Panicle length/cm | 23.05±0.91 | 21.33±1.15 |
| 有效穗数 No. of effective panicles | 8.3±0.5 | 8.0±0.8 |
| 抽穗期 Heading date/d | 98.0±0.8 | 147.3±2.5** |
| 每穗实粒数 No. of filled grains per panicle | 171.5±4.0 | 173.0±2.7 |
| 结实率 Seed-setting rate/% | 93.72±0.01 | 92.20±0.01 |
| 千粒重 1000-grain weight/g | 31.50±0.57 | 31.57±0.40 |
| 一次枝梗数 Primary rachis branch number | 12.0±0.7 | 12.6±0.6 |
| 二次枝梗数 Secondary rachis branch number | 36.8±1.3 | 37.3±0.6 |
| 组合 Cross | F1表型 Phenotype of F1 | F2 | χ2 | ||
|---|---|---|---|---|---|
| 正常植株数 No. of normal plants | 迟抽穗植株数 No. of heading- delayed plants | 总数 Total | |||
| DTH9/93-11 | 正常抽穗期 Normal | 662 | 208 | 870 | 0.281 |
| 93-11/DTH9 | 正常抽穗期 Normal | 389 | 119 | 508 | 0.343 |
表4 迟抽穗突变体dth9的遗传分析
Table 4 Genetic analysis of the dth9 mutant.
| 组合 Cross | F1表型 Phenotype of F1 | F2 | χ2 | ||
|---|---|---|---|---|---|
| 正常植株数 No. of normal plants | 迟抽穗植株数 No. of heading- delayed plants | 总数 Total | |||
| DTH9/93-11 | 正常抽穗期 Normal | 662 | 208 | 870 | 0.281 |
| 93-11/DTH9 | 正常抽穗期 Normal | 389 | 119 | 508 | 0.343 |
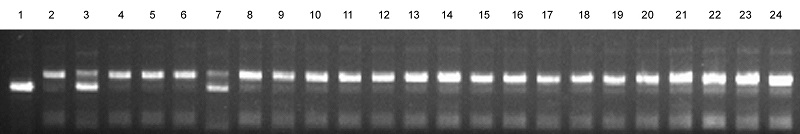
图2 利用标记RM444对F2 群体中21个突变体单株进行基因型分析 1-日本晴; 2-93-11; 3-F1 ; 4~24-F2 群体中突变体表型的单株; 7-单交换单株。
Fig. 2. Genotype analysis of the 21 F2 plants with mutant phenotype using the marker RM444. 1, Nipponbare; 2, 93-11; 3, F1; 4-24, Individuals with mutant phenotype in the F2 population; 7, Single crossing-over plant.
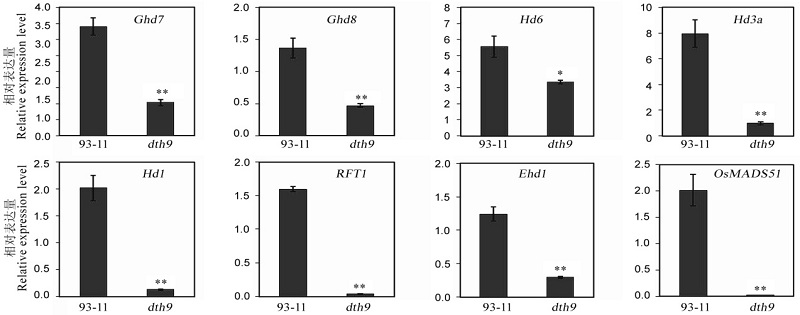
图4 抽穗期相关基因在dth9和野生型中的表达差异数据用平均数±标准差表示(n=3)。*,** 分别表示在0.05和0.01水平上差异显著(t检验)。
Fig. 4. Expression levels of genes associated with heading date in the dth9 mutant and the wild-type. Figures were shown as mean ± SD (n=3). *, ** Significant at 0.05 and 0.01 levels by t-test, respectively.
| [1] | 刘广林, 罗群昌, 陈远孟, 等. 水稻种质资源抽穗扬花期耐冷性鉴定评价. 西南农业学报, 2013, 26(2): 395-398. |
| Liu G L, Luo Q C, Chen Y M, et al.Analysis and evaluation on cold tolerance for rice germplasm resources at flowering stage.Southwest China J Agric Sci, 2013, 26(2): 395-398.(in Chinese with English abstract) | |
| [2] | 邱磊, 蒋海潮, 冯玉涛, 等.控制水稻抽穗期和株高的QTL定位及遗传分析. 基因组学与应用生物学, 2014, 33(4): 828-835 |
| Qiu L, Jiang H C,Feng Y T, et al.Mapping and genetic analycis of QTL for heading date and plant height in rice.Genonm Appl Biol,2014,33(4):828-835.(in Chinese with English abstract) | |
| [3] | 邓晓建, 周开达, 李仁端, 等. 水稻完全显性早熟性的发现和基因定位. 中国农业科学, 2001, 34(3): 233-239. |
| Deng X J, Zhou K D, Li R D, et al.Identification and gene mapping of completely dominant earliness in rice (Oryza sativa L.).Sci Agric Sin, 2001, 34(3): 233-239.(in Chinese with English abstract) | |
| [4] | 胡时开, 苏岩, 叶卫军, 等. 水稻抽穗期遗传与分子调控机理研究进展. 中国水稻科学, 2012, 26(3): 373-382. |
| Hu S K, Sun Y, Ye W J, et al.Advances in genetic analysis and molecular regulation mechanism of heading date in rice (Oryza sativa L.).Chin J Rice Sci, 2012, 26(3): 373-382.(in Chinese with English abstract) | |
| [5] | Yano M, Katayose Y, Ashikari M, et al.Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS,Plant Cell, 2000, 12(12): 2473-2484. |
| [6] | Kojima S, Takahashi Y, Kobayashi Y, et al.Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions.Plant Cell Physiol, 2002, 43(10): 1096-1105. |
| [7] | Doi K, Izawa T, Fuse T, et al.Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1.Genes & Dev, 2004, 18(8): 926-936. |
| [8] | Takahashi Y, Shomura A, Sasaki T, et al.Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2.Proc Natl Acad Sci USA, 2001, 98(14): 7922-7927. |
| [9] | Matsubara K, Yamanouchi U, Nonoue Y, et al.Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering.Plant J, 2011, 66(4): 603-612. |
| [10] | Gao H, Zheng X M, Fei G, et al.Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice.Plos Genet, 2013, 9(2): e1003281. |
| [11] | Xue W, Xing Y, Weng X, et al.Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice.Nat Genet, 2008, 40(6): 761-767. |
| [12] | Wei X, Xu J, Guo H, et al.DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously.Plant Physiol, 2010, 153(4): 1747-1758. |
| [13] | Wu K S, Tanksley S D.Abundance, polymorphism and genetic mapping of microsatellites in rice.Mol Gen Genet, 1993, 241(1): 225-235. |
| [14] | Livak K J, Schmittgen T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method.Methods, 2001, 25: 402-408. |
| [15] | Hirochika H, Guiderdoni E, An G, et al.Rice mutant resources for gene discovery.Plant Mol Biol, 2004, 54(3): 325-334. |
| [16] | 郭梁, 张振华, 庄杰云. 水稻抽穗期QTL及其与产量性状遗传控制的关系. 中国水稻科学, 2012, 26(2): 235-245. |
| Guo L, Zhang Z H, Zhuang J Y.Quantiative trait loci for heading date and their relationship with the genetic control of yield traits in rice (Oryza sativa).Chin J Rice Sci, 2012, 26(2): 235-245.(in Chinese with English abstract) | |
| [17] | Thomson M J, Edwards J D, Septiningsih E M, et a1. Substitution mapping of dth1.1, a flowering-time quantitative trait locus (QTL) associated with transgressive variation in rice, reveals multiple sub-QTL.Genetics, 2006, 172: 2501-2514. |
| [18] | Lin H, Ashikari M, Yamanouchi U, et a1. Identification and characterization of a quantitative trait locus, Hd9, controlling heading date in rice.Breeding Sci, 2002, 52(1): 35-41. |
| [19] | Takeuchi Y, Lin S Y, Sasaki T, et a1. Fine linkage mapping enables dissection of closely linked quantitative trait loci for seed dormancy and heading in rice.Theor Appl Genet, 2003, 107(7): 1174-1180. |
| [20] | Matsubara K, Kono I, Hori K, et a1. Novel QTLs for photoperiodic flowering revealed by using reciprocal backcross inbred lines from crosses between japonica rice cultivars.Theor Appl Genet, 2008, 117(6): 935-945. |
| [21] | Monna L, Lin H X, Kojima S, et a1. Genetic dissection of a genomic region for a quantitative trait locus, Hd3, into two loci, Hd3a and Hd3b, controlling heading date in rice.Theor Appl Genet, 2002, 104(5): 772-778. |
| [22] | Lin H, Liang Z W, Sasaki T, et a1. Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice.Breeding Sci, 2003, 53(1): 51-59. |
| [23] | Yamamoto T, Kuboki Y, Lin S Y, et a1. Fine mapping of quantitative trait loci Hd-1, Hd-2 and Hd-3, controlling heading date of rice, as single Mendelian factors.Theor Appl Genet, 1998, 97(1): 37-44. |
| [24] | Yan W H, Wang P, Chen H X, et al.A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice.Mol Plant, 2011, 4(2): 319-330. |
| [25] | 杜雪树, 戚华雄, 廖世勇, 等.水稻抽穗期分子生物学研究进展. 湖北农业科学, 2013,52(24): 5958-5962. |
| Du X S,Qi H X,Liao S Y, et al.Advances on the molecular biology of rice heading date.Hubei Agric Sci,2013,52(24):5958-5962.(in Chinese) |
| [1] | 郭展, 张运波. 水稻对干旱胁迫的生理生化响应及分子调控研究进展[J]. 中国水稻科学, 2024, 38(4): 335-349. |
| [2] | 韦还和, 马唯一, 左博源, 汪璐璐, 朱旺, 耿孝宇, 张翔, 孟天瑶, 陈英龙, 高平磊, 许轲, 霍中洋, 戴其根. 盐、干旱及其复合胁迫对水稻产量和品质形成影响的研究进展[J]. 中国水稻科学, 2024, 38(4): 350-363. |
| [3] | 许丹洁, 林巧霞, 李正康, 庄小倩, 凌宇, 赖美玲, 陈晓婷, 鲁国东. OsOPR10正调控水稻对稻瘟病和白叶枯病的抗性[J]. 中国水稻科学, 2024, 38(4): 364-374. |
| [4] | 候小琴, 王莹, 余贝, 符卫蒙, 奉保华, 沈煜潮, 谢杭军, 王焕然, 许用强, 武志海, 王建军, 陶龙兴, 符冠富. 黄腐酸钾提高水稻秧苗耐盐性的作用途径分析[J]. 中国水稻科学, 2024, 38(4): 409-421. |
| [5] | 胡继杰, 胡志华, 张均华, 曹小闯, 金千瑜, 章志远, 朱练峰. 根际饱和溶解氧对水稻分蘖期光合及生长特性的影响[J]. 中国水稻科学, 2024, 38(4): 437-446. |
| [6] | 刘福祥, 甄浩洋, 彭焕, 郑刘春, 彭德良, 文艳华. 广东省水稻孢囊线虫病调查与鉴定[J]. 中国水稻科学, 2024, 38(4): 456-461. |
| [7] | 陈浩田, 秦缘, 钟笑涵, 林晨语, 秦竞航, 杨建昌, 张伟杨. 水稻根系和土壤性状与稻田甲烷排放关系的研究进展[J]. 中国水稻科学, 2024, 38(3): 233-245. |
| [8] | 缪军, 冉金晖, 徐梦彬, 卜柳冰, 王平, 梁国华, 周勇. 过量表达异三聚体G蛋白γ亚基基因RGG2提高水稻抗旱性[J]. 中国水稻科学, 2024, 38(3): 246-255. |
| [9] | 尹潇潇, 张芷菡, 颜绣莲, 廖蓉, 杨思葭, 郭岱铭, 樊晶, 赵志学, 王文明. 多个稻曲病菌效应因子的信号肽验证和表达分析[J]. 中国水稻科学, 2024, 38(3): 256-265. |
| [10] | 朱裕敬, 桂金鑫, 龚成云, 罗新阳, 石居斌, 张海清, 贺记外. 全基因组关联分析定位水稻分蘖角度QTL[J]. 中国水稻科学, 2024, 38(3): 266-276. |
| [11] | 魏倩倩, 汪玉磊, 孔海民, 徐青山, 颜玉莲, 潘林, 迟春欣, 孔亚丽, 田文昊, 朱练峰, 曹小闯, 张均华, 朱春权. 信号分子硫化氢参与硫肥缓解铝对水稻生长抑制作用的机制[J]. 中国水稻科学, 2024, 38(3): 290-302. |
| [12] | 周甜, 吴少华, 康建宏, 吴宏亮, 杨生龙, 王星强, 李昱, 黄玉峰. 不同种植模式对水稻籽粒淀粉含量及淀粉关键酶活性的影响[J]. 中国水稻科学, 2024, 38(3): 303-315. |
| [13] | 关雅琪, 鄂志国, 王磊, 申红芳. 影响中国水稻生产环节外包发展因素的实证研究:基于群体效应视角[J]. 中国水稻科学, 2024, 38(3): 324-334. |
| [14] | 许用强, 姜宁, 奉保华, 肖晶晶, 陶龙兴, 符冠富. 水稻开花期高温热害响应机理及其调控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 111-126. |
| [15] | 吕海涛, 李建忠, 鲁艳辉, 徐红星, 郑许松, 吕仲贤. 稻田福寿螺的发生、危害及其防控技术研究进展[J]. 中国水稻科学, 2024, 38(2): 127-139. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||