
Chinese Journal OF Rice Science ›› 2025, Vol. 39 ›› Issue (6): 760-770.DOI: 10.16819/j.1001-7216.2025.240707
• Research Papers • Previous Articles Next Articles
CHEN Wei1,3, YE Yuanmei1, ZHAO Jianhua1, FENG Zhiming1,2, CHEN Zongxiang1,2, HU Keming1,2, ZUO Shimin1,2,*( )
)
Received:2024-07-09
Revised:2024-08-12
Online:2025-11-10
Published:2025-11-19
Contact:
ZUO Shimin
陈伟1,3, 叶元妹1, 赵剑华1, 冯志明1,2, 陈宗祥1,2, 胡珂鸣1,2, 左示敏1,2,*( )
)
通讯作者:
左示敏
基金资助:CHEN Wei, YE Yuanmei, ZHAO Jianhua, FENG Zhiming, CHEN Zongxiang, HU Keming, ZUO Shimin. Modifying Heading Date of Nanjing 46 via CRISPR/Cas9-mediated Genome Editing[J]. Chinese Journal OF Rice Science, 2025, 39(6): 760-770.
陈伟, 叶元妹, 赵剑华, 冯志明, 陈宗祥, 胡珂鸣, 左示敏. 利用CRISPR/Cas9技术改良南粳46抽穗期[J]. 中国水稻科学, 2025, 39(6): 760-770.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.ricesci.cn/EN/10.16819/j.1001-7216.2025.240707
| 引物名称 Primer name | 引物序列(5'-3') Primer sequence(5'-3') | 用途 Purpose |
|---|---|---|
| DTH8-gF-1 | GGCACGTGAGCCGCATCATGAAGCGG | 构建 DTH8靶点1敲除载体 Construction of DTH8 target 1 knockout vector |
| DTH8-gR-1 | AAACCCGCTTCATGATGCGGCTCACG | |
| DTH8-gF-2 | GGCAAAGATCTCCAAGGAGTCGAAGG | 构建 DTH8靶点2敲除载体 Construction of DTH8 target 2 knockout vector |
| DTH8-gR-2 | AAACCCTTCGACTCCTTGGAGATCTT | |
| Ghd2-gF | GGCAGCGTCGGGCAGCTGCTCTCGAGG | 构建 Ghd2敲除载体 Construction of Ghd2 knockout vector |
| Ghd2-gR | AAACCCTCGAGAGCAGCTGCCCGACGC | |
| Hpt-F | CGGAAGTGCTTGACATTG | 转基因检测 Transgenic detection |
| Hpt-R | GACCTCGTATTGGGAATCC | |
| sgRNA-F | AAGGAATCTTTAAACATACGAACAGATC | 单克隆测序鉴定 Monoclonal sequencing identification Sequencing for Monoclonal |
| Cas9-F | ACTCGAACGCGACCAACTTA | 载体检测 Vector assay |
| Cas9-R | ATCTGCAGAATTGCCCTTCG | |
| DTH8-F | CGAAGGAGCAGGACAGGTTC | DTH8 敲除靶点测序鉴定 Sequencing for DTH8 knockout target |
| DTH8-R | TTGATGGTCTTCCGCTTCTCG | |
| Ghd2-F | TGACGCCTTCCTGTGCCAGGGGTG | Ghd2 敲除靶点测序鉴定 Sequencing for Ghd2 knockout target |
| Ghd2-R | GTCCGACGACTCCACCGCCATCTC |
Table 1. Primers used in this research
| 引物名称 Primer name | 引物序列(5'-3') Primer sequence(5'-3') | 用途 Purpose |
|---|---|---|
| DTH8-gF-1 | GGCACGTGAGCCGCATCATGAAGCGG | 构建 DTH8靶点1敲除载体 Construction of DTH8 target 1 knockout vector |
| DTH8-gR-1 | AAACCCGCTTCATGATGCGGCTCACG | |
| DTH8-gF-2 | GGCAAAGATCTCCAAGGAGTCGAAGG | 构建 DTH8靶点2敲除载体 Construction of DTH8 target 2 knockout vector |
| DTH8-gR-2 | AAACCCTTCGACTCCTTGGAGATCTT | |
| Ghd2-gF | GGCAGCGTCGGGCAGCTGCTCTCGAGG | 构建 Ghd2敲除载体 Construction of Ghd2 knockout vector |
| Ghd2-gR | AAACCCTCGAGAGCAGCTGCCCGACGC | |
| Hpt-F | CGGAAGTGCTTGACATTG | 转基因检测 Transgenic detection |
| Hpt-R | GACCTCGTATTGGGAATCC | |
| sgRNA-F | AAGGAATCTTTAAACATACGAACAGATC | 单克隆测序鉴定 Monoclonal sequencing identification Sequencing for Monoclonal |
| Cas9-F | ACTCGAACGCGACCAACTTA | 载体检测 Vector assay |
| Cas9-R | ATCTGCAGAATTGCCCTTCG | |
| DTH8-F | CGAAGGAGCAGGACAGGTTC | DTH8 敲除靶点测序鉴定 Sequencing for DTH8 knockout target |
| DTH8-R | TTGATGGTCTTCCGCTTCTCG | |
| Ghd2-F | TGACGCCTTCCTGTGCCAGGGGTG | Ghd2 敲除靶点测序鉴定 Sequencing for Ghd2 knockout target |
| Ghd2-R | GTCCGACGACTCCACCGCCATCTC |
| 靶点 Target | 潜在脱靶序列 Off-target sequence | 脱靶指数 Off-score | 潜在基因 Gene | 区域 Region |
|---|---|---|---|---|
| DTH8-Target1 | ACATCAGCCGCATCATGAAGAAG | 0.153 | LOC_Os01g61810 | CDS |
| ACATCAGCCGCATCATGAAGAAG | 0.153 | LOC_Os05g38820 | CDS | |
| ACATGAGCAGCATCAAGAAGGAG | 0.152 | LOC_Os02g16909 | Utr | |
| TCGTGAGTCGCTTCATGGAGCGG | 0.117 | LOC_Os08g36774 | Intron | |
| DTH8-Target2 | CAAGAGCTCCAGGGAGACGGAGG | 0.168 | LOC_Os08g13020 | Intron |
| CAAAATCCCCAAGGAGTAGACAG | 0.126 | LOC_Os07g46910 | CDS | |
| CAAGATCTCCAAAGTATTGATGG | 0.119 | Null | Intergenic | |
| GGAGCTCTCCAATGAGTCGATGG | 0.110 | LOC_Os01g32660 | Intron | |
| Ghd2-Target | CCTAGCCGAGCATCAAAACCGGG | 0.270 | Null | Intergenic |
| GCGTCGTGCAGCTGGTCTCGCGG | 0.197 | LOC_Os01g03420 | CDS | |
| GCGTCAGGGAGCTGCTATCGAGG | 0.157 | LOC_Os04g43350 | CDS | |
| GCCTCCGGCTGCTGATCTCGTGG | 0.143 | LOC_Os05g45430 | CDS |
Table 2. Potential off-target analysis
| 靶点 Target | 潜在脱靶序列 Off-target sequence | 脱靶指数 Off-score | 潜在基因 Gene | 区域 Region |
|---|---|---|---|---|
| DTH8-Target1 | ACATCAGCCGCATCATGAAGAAG | 0.153 | LOC_Os01g61810 | CDS |
| ACATCAGCCGCATCATGAAGAAG | 0.153 | LOC_Os05g38820 | CDS | |
| ACATGAGCAGCATCAAGAAGGAG | 0.152 | LOC_Os02g16909 | Utr | |
| TCGTGAGTCGCTTCATGGAGCGG | 0.117 | LOC_Os08g36774 | Intron | |
| DTH8-Target2 | CAAGAGCTCCAGGGAGACGGAGG | 0.168 | LOC_Os08g13020 | Intron |
| CAAAATCCCCAAGGAGTAGACAG | 0.126 | LOC_Os07g46910 | CDS | |
| CAAGATCTCCAAAGTATTGATGG | 0.119 | Null | Intergenic | |
| GGAGCTCTCCAATGAGTCGATGG | 0.110 | LOC_Os01g32660 | Intron | |
| Ghd2-Target | CCTAGCCGAGCATCAAAACCGGG | 0.270 | Null | Intergenic |
| GCGTCGTGCAGCTGGTCTCGCGG | 0.197 | LOC_Os01g03420 | CDS | |
| GCGTCAGGGAGCTGCTATCGAGG | 0.157 | LOC_Os04g43350 | CDS | |
| GCCTCCGGCTGCTGATCTCGTGG | 0.143 | LOC_Os05g45430 | CDS |
| 靶位点 Target | 阳性株数Positive plants | 突变率 Mutation ratio(%) | 突变基因型比率 Mutant genotype ratio(%) | 突变类型比率 Ratio of mutation types(%) | ||||
|---|---|---|---|---|---|---|---|---|
| 纯合突变率Homozygous | 杂合突变率Heterozygous | 双等位突变率 Bi-allele | 碱基插入 Insert | 碱基缺失Deletion | ||||
| DTH8 Target 1 | 36 | 36.1 | 30.8 | 15.4 | 53.8 | 0.0 | 100.0 | |
| DTH8 Target 2 | 36 | 44.4 | 31.3 | 18.8 | 50.0 | 100.0 | 0.0 | |
| Ghd2 Target | 25 | 84.0 | 28.6 | 23.8 | 47.6 | 9.5 | 90.5 | |
Table 3. Ratios of mutant genotype and mutation types in T0 transgenic plants
| 靶位点 Target | 阳性株数Positive plants | 突变率 Mutation ratio(%) | 突变基因型比率 Mutant genotype ratio(%) | 突变类型比率 Ratio of mutation types(%) | ||||
|---|---|---|---|---|---|---|---|---|
| 纯合突变率Homozygous | 杂合突变率Heterozygous | 双等位突变率 Bi-allele | 碱基插入 Insert | 碱基缺失Deletion | ||||
| DTH8 Target 1 | 36 | 36.1 | 30.8 | 15.4 | 53.8 | 0.0 | 100.0 | |
| DTH8 Target 2 | 36 | 44.4 | 31.3 | 18.8 | 50.0 | 100.0 | 0.0 | |
| Ghd2 Target | 25 | 84.0 | 28.6 | 23.8 | 47.6 | 9.5 | 90.5 | |

Fig. 3. PCR screening of mutant plants without exogenous components M, DNA marker; WT, Positive control; 1−3, DTH8 mutation plants; 4−6, Ghd2 mutation plants.
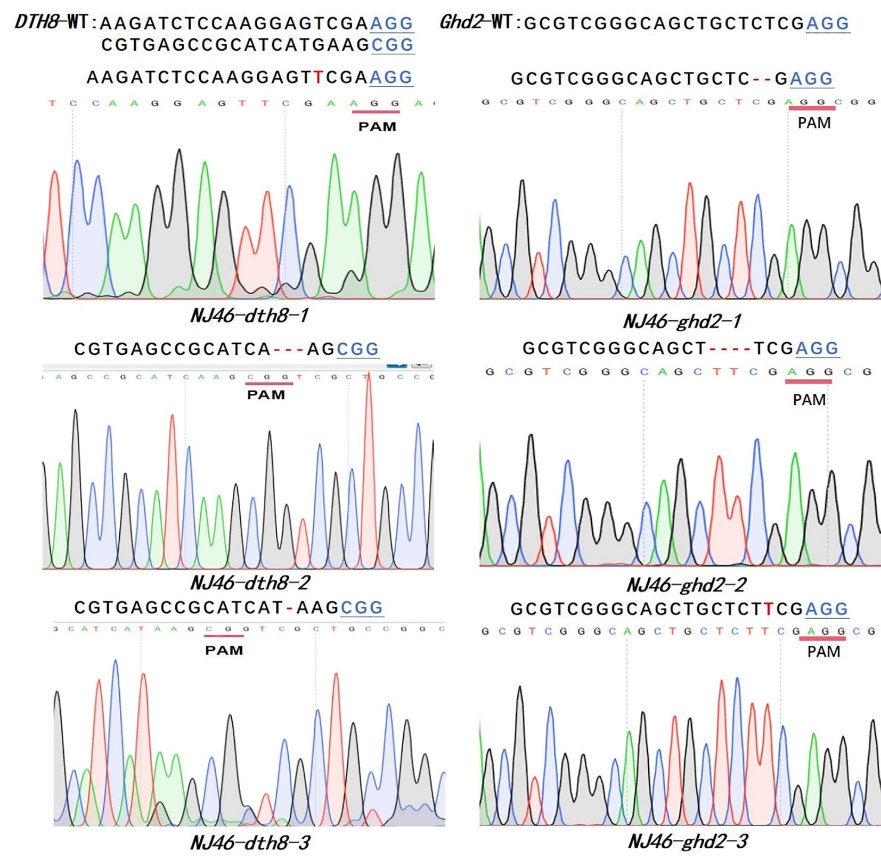
Fig. 4. Six mutation types of homozygotes in T1 Inserted bases are marked in red, deletions are indicated by red dashed lines, and the underlined blue bases represent PAM sequences.
| T1代基因型 Genotype of T1 generation | T2代分离群体抽穗期 Heading date of T2 segregating population plants(d) | 1:3分离比的渐进显著性 Progressive significance of the separation ratio of 1:3 | ||
|---|---|---|---|---|
| 早 Earlier | 无变化 No change | 晚 Later | ||
| Aa1 | 44 | 142 | 0 | 0.672 |
| Aa2 | 62 | 171 | 0 | 0.570 |
| Aa3 | 56 | 162 | 0 | 0.815 |
| Bb1 | 53 | 170 | 0 | 0.671 |
| Bb2 | 47 | 152 | 0 | 0.653 |
| Bb3 | 62 | 200 | 0 | 0.618 |
Table 4. χ2 test of NJ46 mutants phenotype in T2 segregation population
| T1代基因型 Genotype of T1 generation | T2代分离群体抽穗期 Heading date of T2 segregating population plants(d) | 1:3分离比的渐进显著性 Progressive significance of the separation ratio of 1:3 | ||
|---|---|---|---|---|
| 早 Earlier | 无变化 No change | 晚 Later | ||
| Aa1 | 44 | 142 | 0 | 0.672 |
| Aa2 | 62 | 171 | 0 | 0.570 |
| Aa3 | 56 | 162 | 0 | 0.815 |
| Bb1 | 53 | 170 | 0 | 0.671 |
| Bb2 | 47 | 152 | 0 | 0.653 |
| Bb3 | 62 | 200 | 0 | 0.618 |

Fig. 6. Phenotypes of DTH8 and Ghd2 mutants in heading stage (Bar=30 cm) A, Phenotypes of DTH8 and Ghd2 mutants (NJ46-dth8 and NJ46-ghd2, respectively) at heading stage in Yangzhou along the Yangtze River; B, Days to heading of NJ46-dth8 and NJ46-ghd2 mutants in Yangzhou along the Yangtze River; C, Phenotypes of NJ46-dth8 and NJ46-ghd2 mutants at heading stage in Gaoyou; D, Days to heading of NJ46-dth8 and NJ46-ghd2 in Gaoyou; E, Phenotypes of NJ46-dth8 and NJ46-ghd2 mutants at heading stage in Suqian; F, Days to heading of NJ46-dth8 and NJ46-ghd2 mutants in Suqian. Values are shown as mean ± SD. Different letters indicate significant difference among different lines at P < 0.05 according to LSD multiple range test.
| 地点 Site | 株系 Line | 株高 Plant height (cm) | 穗长 Panicle length (cm) | 单株有效穗 Effective panicle per plant | 每穗实粒数 Number of grains per panicle | 千粒重 1000-grain weigh(g) | 单株产量 Yield per plant(g) |
|---|---|---|---|---|---|---|---|
| 高邮Gaoyou | NJ9108 | 96.31±0.74 b | 16.82±0.41 a | 15.52±0.87 a | 142.10±5.81 a | 27.50±0.01 a | 60.63±2.18 a |
| NJ46-ghd2-1 | 90.23±2.23 a | 16.54±0.62 a | 16.25±1.01 b | 148.33±3.21 b | 28.51±0.05 b | 68.51±3.32 b | |
| NJ46-ghd2-2 | 91.64±1.14 a | 16.13±0.45 a | 16.72±1.51 b | 146.22±7.08 ab | 28.32±0.03 b | 69.10±4.23 b | |
| NJ46-ghd2-3 | 91.12±1.43 a | 16.64±0.32 a | 15.61±1.11 ab | 152.24±3.21 b | 28.13±0.02 ab | 66.74±3.61 b | |
| 宿迁 Suqian | NJ518 | 100.20±1.14 b | 17.80±0.36 b | 15.00±1.51 b | 155.31±5.81 b | 27.80±0.03 a | 64.82±4.21 b |
| NJ46-dth8-1 | 98.55±2.32 b | 18.31±0.28 b | 14.23±0.87 a | 156.24±5.77 b | 28.31±0.02 a | 62.82±3.32 b | |
| NJ46-dth8-2 | 98.12±0.74 b | 18.62±0.28 b | 14.94±0.87 b | 151.35±5.77 b | 28.12±0.03 a | 63.34±2.18 b | |
| NJ46-dth8-3 | 89.30±0.74 a | 15.53±0.30 a | 14.41±0.87 a | 122.11±5.77 a | 30.52±0.01 b | 53.61±2.18 a |
Table 5. Comparison of agronomic traits of mutants with NJ9108 and NJ518
| 地点 Site | 株系 Line | 株高 Plant height (cm) | 穗长 Panicle length (cm) | 单株有效穗 Effective panicle per plant | 每穗实粒数 Number of grains per panicle | 千粒重 1000-grain weigh(g) | 单株产量 Yield per plant(g) |
|---|---|---|---|---|---|---|---|
| 高邮Gaoyou | NJ9108 | 96.31±0.74 b | 16.82±0.41 a | 15.52±0.87 a | 142.10±5.81 a | 27.50±0.01 a | 60.63±2.18 a |
| NJ46-ghd2-1 | 90.23±2.23 a | 16.54±0.62 a | 16.25±1.01 b | 148.33±3.21 b | 28.51±0.05 b | 68.51±3.32 b | |
| NJ46-ghd2-2 | 91.64±1.14 a | 16.13±0.45 a | 16.72±1.51 b | 146.22±7.08 ab | 28.32±0.03 b | 69.10±4.23 b | |
| NJ46-ghd2-3 | 91.12±1.43 a | 16.64±0.32 a | 15.61±1.11 ab | 152.24±3.21 b | 28.13±0.02 ab | 66.74±3.61 b | |
| 宿迁 Suqian | NJ518 | 100.20±1.14 b | 17.80±0.36 b | 15.00±1.51 b | 155.31±5.81 b | 27.80±0.03 a | 64.82±4.21 b |
| NJ46-dth8-1 | 98.55±2.32 b | 18.31±0.28 b | 14.23±0.87 a | 156.24±5.77 b | 28.31±0.02 a | 62.82±3.32 b | |
| NJ46-dth8-2 | 98.12±0.74 b | 18.62±0.28 b | 14.94±0.87 b | 151.35±5.77 b | 28.12±0.03 a | 63.34±2.18 b | |
| NJ46-dth8-3 | 89.30±0.74 a | 15.53±0.30 a | 14.41±0.87 a | 122.11±5.77 a | 30.52±0.01 b | 53.61±2.18 a |
| 地点 Site | 株系 Line | 整精米率 Rate of milled rice(%) | 垩白粒率 Chalky grain percentage(%) | 垩白度 Chalkiness(%) | 食味值 Edibility value | 直链淀粉含量 Amylose content(%) |
|---|---|---|---|---|---|---|
| 高邮 Gaoyou | NJ9108 | 70.56±0.26 b | 32.91±0.18 c | 7.07±0.03 b | 71.00±1.52 b | 11.45±0.02 b |
| NJ46-ghd2-1 | 72.21±0.29 b | 12.87±0.20 a | 2.60±0.02 a | 68.70±1.46 ab | 10.14±0.03 a | |
| NJ46-ghd2-2 | 64.52±0.33 a | 17.26±0.14 a | 6.95±0.02 b | 68.30±1.38 a | 10.49±0.03 a | |
| NJ46-ghd2-3 | 71.93±0.26 b | 23.82±0.15 ab | 5.40±0.01 ab | 72.00±1.21 c | 10.32±0.04 a | |
| 宿迁 Suqian | NJ518 | 72.22±0.26 a | 22.26±0.15 a | 5.58±0.02 a | 76.70±1.44 b | 10.00±0.02 a |
| NJ46-dth8-1 | 73.04±0.26 b | 26.14±0.14 a | 6.45±0.01 a | 74.30±1.26 b | 10.31±0.02 a | |
| NJ46-dth8-2 | 72.06±0.26 a | 35.72±0.13 b | 9.00±0.02 b | 53.70±2.20 a | 9.81±0.04 a | |
| NJ46-dth8-3 | 72.32±0.26 a | 28.26±0.23 a | 6.60±0.02 a | 60.30±1.65 a | 10.72±0.03 a |
Table 6. Quality comparison of mutants with NJ9108 and NJ518
| 地点 Site | 株系 Line | 整精米率 Rate of milled rice(%) | 垩白粒率 Chalky grain percentage(%) | 垩白度 Chalkiness(%) | 食味值 Edibility value | 直链淀粉含量 Amylose content(%) |
|---|---|---|---|---|---|---|
| 高邮 Gaoyou | NJ9108 | 70.56±0.26 b | 32.91±0.18 c | 7.07±0.03 b | 71.00±1.52 b | 11.45±0.02 b |
| NJ46-ghd2-1 | 72.21±0.29 b | 12.87±0.20 a | 2.60±0.02 a | 68.70±1.46 ab | 10.14±0.03 a | |
| NJ46-ghd2-2 | 64.52±0.33 a | 17.26±0.14 a | 6.95±0.02 b | 68.30±1.38 a | 10.49±0.03 a | |
| NJ46-ghd2-3 | 71.93±0.26 b | 23.82±0.15 ab | 5.40±0.01 ab | 72.00±1.21 c | 10.32±0.04 a | |
| 宿迁 Suqian | NJ518 | 72.22±0.26 a | 22.26±0.15 a | 5.58±0.02 a | 76.70±1.44 b | 10.00±0.02 a |
| NJ46-dth8-1 | 73.04±0.26 b | 26.14±0.14 a | 6.45±0.01 a | 74.30±1.26 b | 10.31±0.02 a | |
| NJ46-dth8-2 | 72.06±0.26 a | 35.72±0.13 b | 9.00±0.02 b | 53.70±2.20 a | 9.81±0.04 a | |
| NJ46-dth8-3 | 72.32±0.26 a | 28.26±0.23 a | 6.60±0.02 a | 60.30±1.65 a | 10.72±0.03 a |
| [1] | 姚伟, 佟越强, 刘棋, 臧华栋, 杨亚东, 戚志强, 曾昭海. 全球水稻生产时空变化特征及贸易趋势分析[J]. 南方农业学报, 2022, 53(6): 1776-1784. |
| Yao W, Tong Y Q, Liu Q, Zang H D, Yang Y D, Qi Z Q, Zeng Z H. Spatiotemporal change characteristics and trade trend of global rice production[J]. Journal of Southern Agriculture, 2022, 53(6): 1776-1784. (in Chinese with English abstract) | |
| [2] | 彭永彬, 杜晨阳, 郑崇珂, 周晋军, 孙伟, 和亚男, 谢先芝. 水稻抽穗期调控基因Hd6的PARMS标记开发与利用[J]. 山东农业科学, 2022, 54(8): 1-6. |
| Peng Y B, Du C Y, Zheng C K, Zhou J J, Sun W, He Y N, Xie X Z. Development and application of PARMS markers specific for rice heading date regulation gene Hd6[J]. Shandong Agricultural Sciences, 2022, 54(8): 1-6. (in Chinese with English abstract) | |
| [3] | Zhou S R, Zhu S S, Cui S, Hou H G, Wu H Q, Hao B Y, Cai L, Xu Z, Liu L L, Jiang L, Wang H Y, Wan J M. Transcriptional and post-transcriptional regulation of heading date in rice[J]. The New Phytologist, 2021, 230(3): 943-956. |
| [4] | Chen R Z, Deng Y W, Ding Y L, Guo J X, Qiu J, Wang B, Wang C S, Xie Y Y, Zhang Z H, Chen J X, Chen L T, Chu C C, He G C, He Z H, Huang X H, Xing Y Z, Yang S H, Xie D X, Liu Y G, Li J Y. Rice functional genomics: Decades’ efforts and roads ahead[J]. Science China: Life Sciences, 2022, 65(1): 33-92. |
| [5] | 李斌. 利用CRISPR/Cas9技术创制抽穗期改良的水稻新种质[D]. 镇江: 江苏大学, 2022. |
| Li B. Development of new rice germplasms with improved heading date via CRISPR/Cas9 technology[D]. Zhenjiang: Jiangsu University, 2022. (in Chinese with English abstract) | |
| [6] | 蒋丹, 洪广成, 陈倩, 刘石锋, 秦小健. 水稻抽穗期分子调控研究进展[J]. 分子植物育种, 2019, 17(21): 7071-7077. |
| Jiang D, Hong G C, Chen Q, Liu S F, Qin X J. Research progress in molecular regulation of heading date in rice (Oryza sativa)[J]. Molecular Plant Breeding, 2019, 17(21): 7071-7077. (in Chinese with English abstract) | |
| [7] | 王婧莹, 赵广欣, 邱冠凯, 方军. 水稻抽穗期途径基因的磷酸化、泛素化研究进展[J]. 中国水稻科学, 2022, 36(3): 215-226. |
| Wang J Y, Zhao G X, Qiu G K, Fang J. Advances in research on the modification of the heading date genes in rice by phosphorylation and ubiquitination pathways[J]. Chinese Journal of Rice Science, 2022, 36(3): 215-226. (in Chinese with English abstract) | |
| [8] | Dai X D, Ding Y N, Tan L B, Fu Y C, Liu F X, Zhu Z F, Sun X Y, Sun X Y, Gu P, Cai H W, Sun C Q. LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice (Oryza rufipogon)[J]. Journal of Integrative Plant Biology, 2012, 54(10): 790-799. |
| [9] | Yan W H, Wang P, Chen H X, Zhou H J, Li Q P, Wang C R, Ding Z H, Zhang Y S, Yu S B, Xing Y Z, Zhang Q F. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice[J]. Molecular Plant, 2011, 4(2): 319-330. |
| [10] | Wei X J, Xu J F, Guo H N, Jiang L, Chen S H, Yu C Y, Zhou Z L, Hu P S, Zhai H Q, Wan J M. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously[J]. Plant Physiology, 2010, 153(4): 1747-1758. |
| [11] | Fan X W, Wang P F, Qi F X, Hu Y, Li S L, Zhang J, Liang L W, Zhang Z Y, Liu J H, Xiong L Z, Xing Y Z. The CCT transcriptional activator Ghd2 constantly delays the heading date by upregulating CO3 in rice[J]. Journal of Genetics and Genomics, 2023, 50(10): 755-764. |
| [12] | 王静毅, 甘珊珊, 贾彩红, 刘菊华. CRISPR/Cas9技术在热带作物育种中的应用研究进展[J]. 植物遗传资源学报, 2024, 25(3): 312-322. |
| Wang J Y, Gan S S, Jia C H, Liu J H. Application of CRISPR/Cas9 technology in tropical crops breeding[J]. Journal of Plant Genetic Resources, 2024, 25(3): 3312-322. (in Chinese with English abstract) | |
| [13] | 顾爽, 郑文静, 马殿荣. CRISPR/Cas9基因编辑系统在水稻育种应用的研究进展[J]. 分子植物育种, 2021, 19(10): 3314-3322. |
| Gu S Z, Zhen W J, M, Ma D R. Research progress ofof CRISPR/Cas9 gene-editing system in rice breeding[J]. Molecular Plant Breeding, 2021, 19(10): 33314-3322. (in Chinese with English abstract) | |
| [14] | Gupta D, Bhattacharjee O, Mandal D, Sen M K, Dey D, Dasgupta A, Kazi T A, Gupta R, Sinharoy S, Acharya K, Chattopadhyay D, Ravichandiran V, Roy S, Ghosh D. CRISPR-Cas9 system: A new-fangled dawn in gene editing[J]. Life Sciences, 2019, 232: 116636. |
| [15] | 林萌萌, 李春娟, 闫彩霞, 孙全喜, 赵小波, 王娟, 苑翠玲, 单世华. CRISPR/Cas9基因编辑技术在作物中的应用[J]. 核农学报, 2021, 35(6): 1329-1339. |
| Lin M M, Li C J, Yan C X, Sun Q X, Zhao X B, Wang J, Yuan C L, Shan S H. Application of CRISPR/Cas9 gene editing technology in crops[J]. Journal of Nuclear Agricultural Sciences, 2021, 35(6): 1329-1339. (in Chinese with English abstract) | |
| [16] | 牛淑琳, 鞠培娜, 周冠华, 戴南平, 周晋军, 谢先芝, 郑崇珂. 利用CRISPR/Cas9技术编辑OsRR22基因创制耐盐水稻种质资源[J]. 山东农业科学, 2023, 55(2): 30-35. |
| Niu S L, Ju P N, Z, Zhou G H, D, Dai N P, Z, Zhou J J, Xie X Z, Zheng C K. Creation of salt-tolerant rice germplasm by editingediting OsRR22 gene via CRISPR/Cas9 technique [e[J]. Shandong Agricultural Sciences, 2023, 55(2): 30-35. (in Chinese with English abstract) | |
| [17] | 李刚, 高清松, 李伟, 张雯霞, 王健, 程保山, 王迪, 高浩, 徐卫军, 陈红旗, 纪剑辉. 定向敲除SD1基因提高水稻的抗倒性和稻瘟病抗性[J]. 中国水稻科学, 2023, 37(4): 359-367. |
| Li G, Gao Q S, Li W, Zhang W W, Wang J, Cheng B S, Wang D, Gao H, Xu W J, Chen H Q, Ji J H. Directed knockout of SD1 gene improves lodging resistance and blast resistance of rice[J]. Chinese Journal of Rice Science, 2023, 37(4): 359-367. (in Chinese with English abstract) | |
| [18] | Zhang C J, Yun P, Xia J F, Zhou K N, Wang L L, Zhang J W, Zhao B, Yin D K, Fu Z, Wang Y L, Ma T C, Li Z F, Wu D X. CRISPR/Cas9-mediated editing of Wx and BADH2 genes created glutinous and aromatic two-line hybrid rice[J]. Molecular Breeding, 2023, 43(4): 24. |
| [19] | Hori K, Ogiso-Tanaka E, Matsubara K, Yamanouchi U, Ebana K, Yano M. Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response[J]. The Plant Journal, 2013, 76(1): 36-46. |
| [20] | Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, Yano M. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering[J]. Plant &Cell Physiology, 2012, 53(4): 709-716. |
| [21] | Xue W Y, Xing Y Z, Weng X Y, Zhao Y, Tang W J, Wang L, Zhou H J, Yu S B, Xu C G, Li X H, Zhang Q F. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice[J]. Nature Genetics, 2008, 40(6): 761-767. |
| [22] | Imran M, Shafiq S, T, Tang X R. CRISPR-Cas9-mediated editing ofof BADH2 gene triggeredtriggered fragrance revolution in rice[J]. Physiologia Plantarum, 2023, 175(1): e13871. |
| [23] | Fiaz S, Ahmad S, Ali Noor M, Wang X K, Younas A, Riaz A, Riaz A, Ali F. Applications of the CRISPR/Cas9 system for rice grain quality improvement: Perspectives and opportunities[J]. International Journal of Molecular Sciences, 2019, 20(4): 888. |
| [24] | Chen H M, Ye R, Liang Y, Zhang S C, Liu X L, Sun C J, Li F B, Yi J C. Generation of low-cadmium rice germplasms via knockout of OsLCD using CRISPR/Cas9[J]. Journal of Environment Science (China), 2023, 126: 138-152. |
| [25] | Sheng X B, Ai Z Y, Tan Y N, Hu Y Y, Guo X Y, Liu X L, Sun Z Z, Yu D, Chen J, Tang N, Duan M J, Yuan D Y.. Novel salinity-tolerant third-generation hybrid rice developed via CRISPR/Cas9-mediated gene editing[J]. International Journal of Molecular Sciences, 2023, 24(9): 8025. |
| [26] | Zhang Y, Lin X F, Li L, Piao R H, Wu S Q, Song A Q, Gao M M, Jin Y M. CRISPR/Cas9-mediated knockout of Bsr-d1 enhances the blast resistance of rice in Northeast China[J]. Plant Cell Reports, 2024, 43(4): 100. |
| [27] | 张浩, 柳絮, 宣宁, 张华, 高瑞钰, 赵倩倩, 姚方印. 利用CRISPR/Cas9技术编辑DTH8基因改良水稻99-25的抽穗期[J]. 华北农学报, 2020, 35(6): 58-66. |
| Zhang H L, Liu X, Xuan N Z, Zhang H, Gao R Y Z, Zhao Q Q, Y, Yao F Y. Editing DTH8 gene using CRISPR/Cas9 technology to improveimprove heading date of rice 99-25[J]. Acta Agriculturae Boreali-Sinica, 2020, 35(6): 58-66. (in Chinese with English abstract) | |
| [28] | Zhou S R, Cai L, Wu H Q, Wang B X, Gu B, Cui S, Huang X L, Xu Z, Hao B Y, Hou H G, Hu Y, Li C, Tian Y L, Liu X, Chen L M, Liu S J, Jiang L, Wan J M. Fine-tuning rice heading date through multiplex editing of the regulatory regions of key genes by CRISPR-Cas9[J]. Plant Biotechnology Journal, 2024, 22(3): 751-758. |
| [29] | Sun K L, Huang M H, Zong W B, Xiao D D, Lei C, Luo Y Q, Song Y G, Li S T, Hao Y, Luo W N, Xu B Q, Guo X T, Wei G L, Chen L T, Liu Y G, Guo J X. Hd1, Ghd7, and DTH8 synergistically determine the rice heading date and yield-related agronomic traits[J]. Journal of Genetics and Genomics, 2022, 49(5): 437-447. |
| [30] | Fujino K. Days to heading, controlled by the heading date genes, Hd1 and DTH8, limits rice yield-related traits in Hokkaido, Japan[J]. Breeding Science, 2020, 70(3): 277-282. |
| [31] | Liu J H, Shen J Q, Xu Y, Li X H, Xiao J H, Xiong L Z. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice[J]. Journal of Experimental Botany, 2016, 67(19): 5785-5798. |
| [32] | 康雪蒙, 薄晋芳, 马梦影, 巩文靓, 姜恭好, 段海燕. 淀粉合成基因与水稻胶稠度、糊化温度和直链淀粉含量相关性分析[J]. 东北农业科学, 2023, 48(1): 4-29. |
| Kang X M, Bo J F, Ma M Y, Gong W J, Jiang G H, Duan H Y. Correlation analysis of starch synthesis genes with rice gel consistency, gelatinization temperature, and amylose content[J]. Journal of Northeast Agricultural Sciences, 2023, 48(1): 4-29. (in Chinese with English abstract) |
| [1] | CHEN Ling, LIN Wenying, LIANG Limei, OUYANG Younan, YE Shenghai, JI Zhijuan. Flowering Habits of Rice and Its Application in Breeding japonica Cytoplasmic Male Sterile Lines [J]. Chinese Journal OF Rice Science, 2025, 39(6): 731-743. |
| [2] | WANG Juan, WU Lijuan, HONG Haibo, YAO Zhiwen, WANG Lei, E Zhiguo. Research Progress on Biological Functions of Ubiquitin-conjugating Enzymes in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(6): 744-750. |
| [3] | TAO Shibo, XU Na, XU Zhengjin, LIU Chang, XU Quan. Cloning of Cold6 Conferring Cold Tolerance in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(6): 751-759. |
| [4] | HOU Guihua, ZHOU Liguo, LEI Jianguo, CHEN Hong, NIE Yuanyuan. Preliminary Analysis of Function and Mechanism of OsRDR5 Gene in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(6): 779-788. |
| [5] | WANG Shilin, WU Ting, ZHOU Shiqi, SONG Siming, HU Biaolin. Identification of QTLs for Seed Storability in Dongxiang Wild Rice by Integrating BSA-Seq and QTL Analysis [J]. Chinese Journal OF Rice Science, 2025, 39(6): 789-800. |
| [6] | LU Shuai, TAO Tao, LIU Ran, ZHOU Wenyu, CAO Lei, YANG Qingqing, ZHANG Mingqiu, REN Xinzhe, YANG Zhidi, XU Fuxiang, HUAN Haidong, GONG Yuanhang, ZHANG Haocheng, JIN Sukui, CAI Xiuling, GAO Jiping, LENG Yujia. Identification and Gene Cloning of a Long Sterile Lemma and Small Grain Mutant lsg8 in Rice (Oryza sativa L.) [J]. Chinese Journal OF Rice Science, 2025, 39(6): 813-824. |
| [7] | DENG Huan, LIU Yapei, WANG Chunlian, GUO Wei, CHEN Xifeng, JI Zhiyuan. Mapping Analysis of a New Bacterial Blight Resistance Gene Xa49(t) in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(6): 825-831. |
| [8] | ZHANG Lanlan, LIU Dilin, MA Xiaozhi, HUO Xing, KONG Le, LI Jinhua, FU Chongyun, LIAO Yilong, ZHU Manshan, ZENG Xueqin, LIU Wuge, WANG Feng. Quality Traits Affecting Eating Quality in indica Rice Under Different Nitrogen Application Levels in Early and Late Seasons in South China [J]. Chinese Journal OF Rice Science, 2025, 39(6): 832-846. |
| [9] | BIAN Jinlong, REN Gaolei, QIU Shi, XU Fangfu, HU Zhonglei, ZHANG Hongcheng, WEI Haiyan. Effect of Application Methods of Mixed Controlled-release Nitrogen Fertilizer on Yield and Nitrogen Utilization of Good Taste Quality japonica Rice Under Different Mechanical Transplanting Methods in the Huaibei Region [J]. Chinese Journal OF Rice Science, 2025, 39(6): 847-862. |
| [10] | LI Xing, ZHANG Ruichun, CHEN Ge, XIE Jiaxin, XIAO Zhengwu, CAO Fangbo, CHEN Jiana, HUANG Min. Yield Formation and Photosynthetic Characteristics of Machine-transplanted Late-season Rice with Short Growth Duration [J]. Chinese Journal OF Rice Science, 2025, 39(6): 863-872. |
| [11] | LUO Zizi, ZHANG Dejun, CHEN Dongdong, BI Miao, ZHU Yuhan, HAN Xu, WU Qiang, LI Yuechen. Characteristics of Spatiotemporal Evolution of Thermal Resources in Ratoon Rice at Heading and Grain-filling Stages in Sichuan Basin in 1981−2022 [J]. Chinese Journal OF Rice Science, 2025, 39(6): 873-886. |
| [12] | HAO Wenqian, CAI Xingjing, YANG Haidong, WU Yuyang, TENG Xuan, XUE Chao, GONG Zhiyun. Advances in Roles of Different Types of Histone Modifications in Responses of Rice to Abiotic Stresses [J]. Chinese Journal OF Rice Science, 2025, 39(5): 575-585. |
| [13] | LU Tingting, YAN Wenhui, SU Xinquan, ZENG Luohua, HUA Liqin, CHEN Jianghua, FENG Baohua, WANG Yuexing, HU Jiang, FU Guanfu. Research Progress on Physiological and Ecological Mechanisms and Regulation Pathways of Yield, Quality and Stress Resistance Response in Perennial Rice [J]. Chinese Journal OF Rice Science, 2025, 39(5): 586-600. |
| [14] | WU Wanting, XU Qian, LIU Dantong, ZHU Changjin, DU Haotian, JU Haoran, HUO Zhongyang, DAI Qigen, LI Guohui, XU Ke. Research Progress in Regulation of Anthocyanin Accumulation in Colored Rice [J]. Chinese Journal OF Rice Science, 2025, 39(5): 601-614. |
| [15] | WANG Jingbo, SU Chang, FENG Jing, JIANG Sixu, XU Hai, CUI Zhibo, ZHAO Minghui. Functional Study on Aluminum Tolerance of OsAlR1 Gene in Rice [J]. Chinese Journal OF Rice Science, 2025, 39(5): 615-623. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||