
中国水稻科学 ›› 2023, Vol. 37 ›› Issue (2): 200-212.DOI: 10.16819/j.1001-7216.2023.220802
王炫栋, 余俊杰, 高润杰, 兰赫婷, 江樱姿, 齐文杰, 宋振, 蒋冬花( )
)
收稿日期:2022-08-04
修回日期:2022-09-05
出版日期:2023-03-10
发布日期:2023-03-10
通讯作者:
蒋冬花
基金资助:
WANG Xuandong, YU Junjie, GAO Runjie, LAN Heting, JIANG Yingzi, QI Wenjie, SONG Zhen, JIANG Donghua( )
)
Received:2022-08-04
Revised:2022-09-05
Online:2023-03-10
Published:2023-03-10
Contact:
JIANG Donghua
摘要:
【目的】评估沙阿霉素链霉菌Sz-11的促生能力和对水稻BLS的生防潜力,探究其初步的抑菌机制。【方法】用牛津杯法测定Sz-11对7种植物病原细菌和11种植物病原真菌的抗菌谱。通过浸种和浇灌试验评估Sz-11的促生潜力。SDS-PAGE电泳、琼脂糖凝胶电泳、CLSM、SEM、FTIR分析发酵液中的活性产物对Xooc蛋白质表达、基因组DNA合成、细胞膜通透性、细胞形态、细胞膜表面物质结构和组成的影响。4个水稻品种的盆栽防效试验,初步明确Sz-11对BLS的生防潜力。【结果】Sz-11对番茄叶斑病菌、苦瓜枯萎病菌、西瓜炭疽病菌、烟草赤星病菌等多种植物致病真菌都具有较好的拮抗活性,对水稻黄单胞菌(Xoo和Xooc)具有突出的拮抗作用。Sz-11能够促进水稻种子萌发生长,缩短萌发周期,加速营养生长期水稻有机质的积累和根系的分化。Sz-11能影响Xooc蛋白表达,改变细胞膜透性和膜表面物质组成,使细胞溶胀、变形、死亡,但不影响Xooc基因组DNA合成。盆栽试验显示,Sz-11对4个水稻品种BLS相对防效高达65.63%~84.38%,且提前预防效果优于发病后治理。【结论】Sz-11具有较广的抗菌谱,是一株有益的植物根际促生菌,通过作用于Xooc细胞膜和影响蛋白质合成来抑制病菌的生长繁殖,能有效防治水稻BLS的发生,具有较好的生防应用潜力。
王炫栋, 余俊杰, 高润杰, 兰赫婷, 江樱姿, 齐文杰, 宋振, 蒋冬花. 一株兼具防病促生功能的沙阿霉素链霉菌Sz-11[J]. 中国水稻科学, 2023, 37(2): 200-212.
WANG Xuandong, YU Junjie, GAO Runjie, LAN Heting, JIANG Yingzi, QI Wenjie, SONG Zhen, JIANG Donghua. Streptomyces zaomyceticus Sz-11, a Potential Biocontrol Agent with the Functions of Preventing Plant Diseases and Promoting Plant Growth[J]. Chinese Journal OF Rice Science, 2023, 37(2): 200-212.
| 菌株 Strain | 侵染植物 Infected plant | 抗性 Resistance |
|---|---|---|
| Xanthomonas oryzae pv. oryzicola, Xooc, RS105 | 水稻Oryza sativa L. | Rif |
| Xanthomonas oryzae pv. oryzae, Xoo, P6 | 水稻Oryza sativa L. | / |
| Pseudomonas syringae pv. tomato, Pst, DC3000 | 番茄Lycopersicon esculentum Miller | / |
| Pseudomonas syringae pv. glycinea, Psg, NJAU | 大豆Glycine max (Linn.) Merr. | Cb |
| Xanthomonas axonopodis pv. phaseoli, Xap, XS2 | 菜豆Phaseolus vulgaris Linn. | Cb |
| Xanthomonas axonopodis pv. glycines, Xag, NEAU001 | 大豆Glycine max (Linn.) Merr. | Cb/Amp |
| Ralstonia solanacearum, FR2 | 烟草Nicotiana tabacum L. | / |
| Gaeumannomyces graminis, MG201 | 小麦Triticum aestivum L. | / |
| Fusarium oxysporum f. sp. momordicae, Fom, FM04 | 苦瓜Momordica charantia L. | / |
| Fusarium oxysporum f. sp. cucumerinum, Foc, FC27 | 黄瓜Cucumis sativus L. | / |
| Fusarium pseudograminearum, FP68 | 小麦Triticum aestivum L. | / |
| Penicillium expansum, MP001 | 苹果Malus pumila Mill. | / |
| Alternaria solani, AS24 | 番茄Lycopersicon esculentum Miller | / |
| Alternaria alternata, AL08 | 烟草Nicotiana tabacum L. | / |
| Alternaria tomato, AT01 | 番茄Lycopersicon esculentum Miller | / |
| Colletotrichum orbiculare, WT204 | 西瓜Citrullus lanatus (Thunb.) Matsum. et Nakai | / |
| Gibberella zeae, GZ69 | 小麦Triticum aestivum L. | / |
| Fusarium solani, FS023 | 杨树Populus L. | / |
表1 抗菌谱试验菌株
Table 1. Strains subjected to antibacterial spectrum test.
| 菌株 Strain | 侵染植物 Infected plant | 抗性 Resistance |
|---|---|---|
| Xanthomonas oryzae pv. oryzicola, Xooc, RS105 | 水稻Oryza sativa L. | Rif |
| Xanthomonas oryzae pv. oryzae, Xoo, P6 | 水稻Oryza sativa L. | / |
| Pseudomonas syringae pv. tomato, Pst, DC3000 | 番茄Lycopersicon esculentum Miller | / |
| Pseudomonas syringae pv. glycinea, Psg, NJAU | 大豆Glycine max (Linn.) Merr. | Cb |
| Xanthomonas axonopodis pv. phaseoli, Xap, XS2 | 菜豆Phaseolus vulgaris Linn. | Cb |
| Xanthomonas axonopodis pv. glycines, Xag, NEAU001 | 大豆Glycine max (Linn.) Merr. | Cb/Amp |
| Ralstonia solanacearum, FR2 | 烟草Nicotiana tabacum L. | / |
| Gaeumannomyces graminis, MG201 | 小麦Triticum aestivum L. | / |
| Fusarium oxysporum f. sp. momordicae, Fom, FM04 | 苦瓜Momordica charantia L. | / |
| Fusarium oxysporum f. sp. cucumerinum, Foc, FC27 | 黄瓜Cucumis sativus L. | / |
| Fusarium pseudograminearum, FP68 | 小麦Triticum aestivum L. | / |
| Penicillium expansum, MP001 | 苹果Malus pumila Mill. | / |
| Alternaria solani, AS24 | 番茄Lycopersicon esculentum Miller | / |
| Alternaria alternata, AL08 | 烟草Nicotiana tabacum L. | / |
| Alternaria tomato, AT01 | 番茄Lycopersicon esculentum Miller | / |
| Colletotrichum orbiculare, WT204 | 西瓜Citrullus lanatus (Thunb.) Matsum. et Nakai | / |
| Gibberella zeae, GZ69 | 小麦Triticum aestivum L. | / |
| Fusarium solani, FS023 | 杨树Populus L. | / |
| 处理组 Treatment groups | 处理方法 Treatment methods |
|---|---|
| 无菌水阴性对照组(CK1) Sterile water group, negative control (CK1) | 无菌剪刀45°剪叶后,喷雾无菌水,并套袋保持剪叶处湿润(24 h) Cut with sterile scissors at 45°followed by sterile water spraying and 20-h bagging to keep them moist by bagging |
| Xooc菌液阳性对照组(CK2) Xooc positive control group (CK2) | 无菌剪刀45°剪叶后,喷雾接种Xooc水悬液,并套袋保持接种部位湿润(24 h) Cut leaves with sterile scissors at 45° followed by Xooc water suspension spraying and 24-h bagging to keep the inoculation site moist |
| 发酵液治疗处理组(T1) Fermentation liquid treatment group (T1) | 无菌剪刀45°剪叶后,喷雾接种Xooc水悬液,套袋保持接种部位湿润;接种3 h后,在叶片正反两面均匀喷洒目标菌株发酵液(剂量:100 µL/cm2),套袋保持接种部位湿润(24 h) Cut leaves with sterile scissors at 45° followed by Xooc water suspension spraying and bagging to keep the inoculation site moist. The fermentation liquid of the target strain was evenly sprayed on both sides of the leaves at a dosage of 100 µL/cm2 3hours after inoculation, and the inoculation site was hugged to keep it moist for 24 hours |
| 发酵液预防处理组(T2) Fermentation liquid prevention treatment group (T2) | 先用喷壶在叶片正反两面均匀喷洒目标菌株发酵液(剂量:100 µL/cm2),套袋保持接种部位湿润;3 h后,无菌剪刀45°剪叶,喷雾接种Xooc水悬液,套袋保持接种部位湿润(24 h) Firstly, the fermentation liquid of the target strain was evenly sprayed on both sides of the leaves with a watering can at the dosage of 100 µL/cm2 3 hours after inoculation, and the inoculation site was bagged to keep it moist. Sterile scissors were used to cut leaves at 45°3 h later, Xooc water suspension was sprayed, and the inoculation site was bagged to make it moist for 24 hours |
| 发酵滤液治疗处理组(T3) Fermentation filtrate treatment group (T3) | 处理方法同T1,但将目标菌株发酵液替换成过滤后不含菌体的发酵滤液 The treatment method was the same as T1, but the fermentation liquid of the target strain was replaced with a fermentation filtrate without bacteria after filtration |
| 发酵滤液预防处理组(T4) Fermentation filtrate prevention treatment group (T4) | 处理方法同T2,但将目标菌株发酵液替换成过滤后不含菌体的发酵滤液 The treatment method was the same as T2, but the fermentation liquid of the target strain was replaced with a fermentation filtrate without bacteria after filtration |
表2 水稻细菌性条斑病防效试验处理方法
Table 2. Treatment methods of rice bacterial leaf streak control effect test.
| 处理组 Treatment groups | 处理方法 Treatment methods |
|---|---|
| 无菌水阴性对照组(CK1) Sterile water group, negative control (CK1) | 无菌剪刀45°剪叶后,喷雾无菌水,并套袋保持剪叶处湿润(24 h) Cut with sterile scissors at 45°followed by sterile water spraying and 20-h bagging to keep them moist by bagging |
| Xooc菌液阳性对照组(CK2) Xooc positive control group (CK2) | 无菌剪刀45°剪叶后,喷雾接种Xooc水悬液,并套袋保持接种部位湿润(24 h) Cut leaves with sterile scissors at 45° followed by Xooc water suspension spraying and 24-h bagging to keep the inoculation site moist |
| 发酵液治疗处理组(T1) Fermentation liquid treatment group (T1) | 无菌剪刀45°剪叶后,喷雾接种Xooc水悬液,套袋保持接种部位湿润;接种3 h后,在叶片正反两面均匀喷洒目标菌株发酵液(剂量:100 µL/cm2),套袋保持接种部位湿润(24 h) Cut leaves with sterile scissors at 45° followed by Xooc water suspension spraying and bagging to keep the inoculation site moist. The fermentation liquid of the target strain was evenly sprayed on both sides of the leaves at a dosage of 100 µL/cm2 3hours after inoculation, and the inoculation site was hugged to keep it moist for 24 hours |
| 发酵液预防处理组(T2) Fermentation liquid prevention treatment group (T2) | 先用喷壶在叶片正反两面均匀喷洒目标菌株发酵液(剂量:100 µL/cm2),套袋保持接种部位湿润;3 h后,无菌剪刀45°剪叶,喷雾接种Xooc水悬液,套袋保持接种部位湿润(24 h) Firstly, the fermentation liquid of the target strain was evenly sprayed on both sides of the leaves with a watering can at the dosage of 100 µL/cm2 3 hours after inoculation, and the inoculation site was bagged to keep it moist. Sterile scissors were used to cut leaves at 45°3 h later, Xooc water suspension was sprayed, and the inoculation site was bagged to make it moist for 24 hours |
| 发酵滤液治疗处理组(T3) Fermentation filtrate treatment group (T3) | 处理方法同T1,但将目标菌株发酵液替换成过滤后不含菌体的发酵滤液 The treatment method was the same as T1, but the fermentation liquid of the target strain was replaced with a fermentation filtrate without bacteria after filtration |
| 发酵滤液预防处理组(T4) Fermentation filtrate prevention treatment group (T4) | 处理方法同T2,但将目标菌株发酵液替换成过滤后不含菌体的发酵滤液 The treatment method was the same as T2, but the fermentation liquid of the target strain was replaced with a fermentation filtrate without bacteria after filtration |
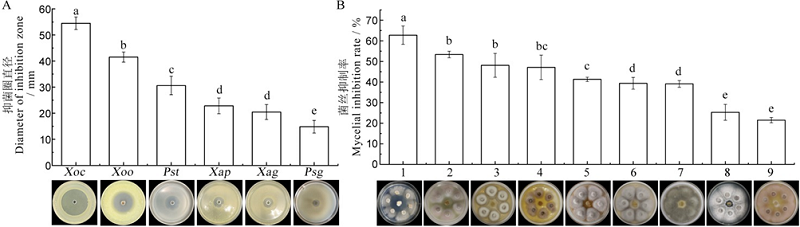
图1 Sz-11菌株抗菌谱 不同小写字母表示处理间差异显著,P<0.05。
Fig. 1. Antibacterial spectrum of strain Sz-11. Different lowercase letters represent significant differences between treatments(P<0.05). 1?Gaeumannomyces graminis, MG201; 2?Fusarium oxysporum f. sp. momordicae, Fom, FM04; 3?Penicillium expansum, MP001; 4?Alternaria solani, AS24; 5?Colletotrichum orbiculare, WT204; 6?Alternaria alternata, AL08; 7?Alternaria tomato, AT01; 8?Fusarium pseudograminearum, FP68; 9?Fusarium oxysporum f. sp. cucumerinum, Foc, FC27.
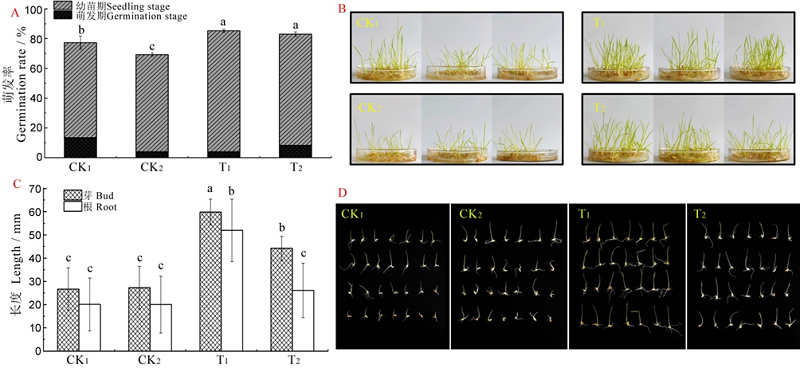
图2 Sz-11发酵液水稻浸种促生试验结果 不同小写字母表示处理间差异显著,P<0.05。
Fig. 2. Results of rice growth promoting experiment by soaking seed with Sz-11 fermentation liquid. Different lowercase letters represent significant differences between the treatments at P<0.05.
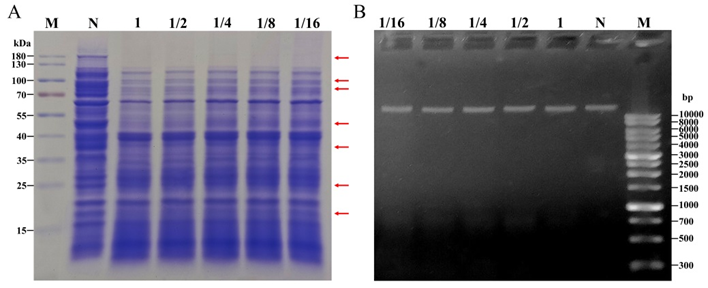
图4 Sz-11发酵滤液处理Xooc的SDS-PAGE蛋白电泳(A)和DNA琼脂糖凝胶电泳(B)结果 M?标记;N?高氏1号培养基对照;1,1/2,1/4,1/8?不同浓度发酵滤液处理。
Fig. 4. Results of SDS-PAGE protein electrophoresis(A) and DNA agarose gel electrophoresis (B) of Xooc treated with Sz-11 fermentation filtrate. M, Marker; N, Gause 1 medium treatment; 1, 1/2, 1/4, 1/8, Different concentrations of fermentation filtrate treatment.
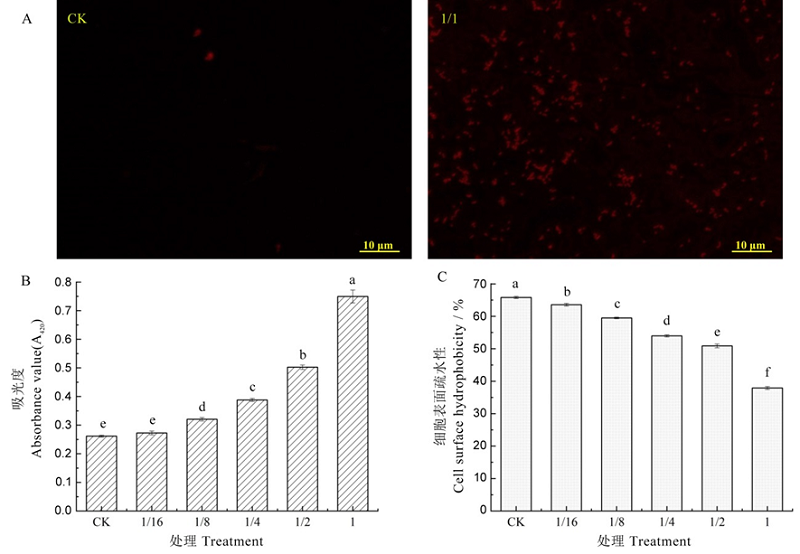
图5 Sz-11发酵滤液处理对Xooc细胞膜通透性及表面疏水性的影响 A?CLSM图;B?Xooc细胞内β-半乳糖苷酶泄露;C?Xooc细胞表面疏水性变化。不同小写字母表示处理间差异显著,P<0.05。
Fig. 5. Effects of Sz-11 fermentation filtrate treatment on cell membrane permeability and surface hydrophobicity of Xooc. A, CLSM figure; B, Xooc intracellular β-galactosidase leakage; C, Changes of hydrophobicity on Xooc cell surface. Different lowercase letters represent significant differences between the treatments at P<0.05.
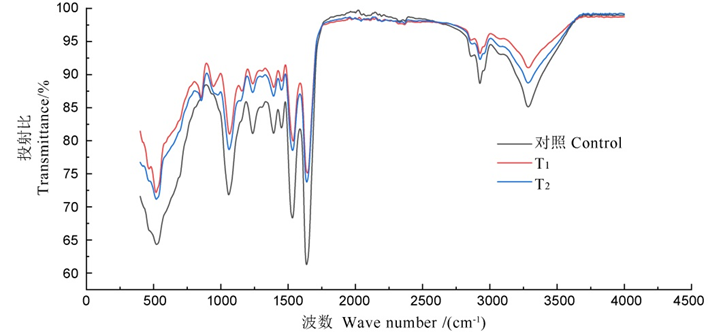
图7 不同浓度Sz-11发酵滤液处理后Xooc细胞的红外吸收光谱 对照?高氏1号培养基处理;T1?2倍稀释发酵滤液处理;T2?4倍稀释发酵滤液处理。
Fig. 7. Infrared absorption spectra of Xooc cell treated with different concentrations of Sz-11 fermentation filtrate. Control, Gause 1 medium treatment; T1, 1/2 fermentation filtrate; T2, 1/4 fermentation filtrate.
| 振动峰分配 Definition of the spectral assignment | 分类 Classification | 振动峰 Peak variation/(cm-1) | ||
|---|---|---|---|---|
| 对照Control | T1 | T2 | ||
| 碳水化合物中的C-O, C-C str, C-O-H, C-O-C伸缩振动峰 C-O, C-C str, C-O-H, C-O-C def of carbohydrates | 糖原和核酸 Glycogen and nucleic acids | 1063.47 | 1063.62 | 1060.50 |
| 磷酸二酯>PO2中P=O的不对称伸缩振动峰 P=O str (asym) of>PO2 phosphodiesters | 主要是核酸 Mainly nucleic acids | 1244.96 | 1239.16 | 1236.03 |
| COO中C=O对称伸缩振动峰 C=O str (sym) of COO | 氨基酸侧链,脂肪酸 Amino acid side chains, fatty acids | 1393.72 | 1393.87 | 1393.75 |
| 酰胺II中蛋白质N-H弯曲振动峰和C-N拉伸振动峰 Amide II (protein N-H bend, C-N stretch) | α螺旋 α helices | 1533.55 | 1539.65 | 1539.50 |
| 酰胺I的β-折叠结构Amide I of β-pleated sheet structures | β-折叠 β-pleated sheet | 1640.66 | 1634.86 | 1634.71 |
| >CH2中C-H不对称伸缩振动峰 C-H str (asym) of>CH2 | 主要是脂类Mainly lipids | 2931.90 | 2926.08 | 2925.95 |
| 羟基O-H伸缩振动峰O-H str of hydroxyl groups | 多糖和蛋白质Polysaccharides, proteins | 3288.93 | 3283.10 | 3274.05 |
表3 不同浓度的Sz-11发酵滤液处理后Xooc细胞振动峰的变化
Table 3. Changes of vibrational peaks of Xooc cell treated with different concentrations of Sz-11 fermentation filtrate.
| 振动峰分配 Definition of the spectral assignment | 分类 Classification | 振动峰 Peak variation/(cm-1) | ||
|---|---|---|---|---|
| 对照Control | T1 | T2 | ||
| 碳水化合物中的C-O, C-C str, C-O-H, C-O-C伸缩振动峰 C-O, C-C str, C-O-H, C-O-C def of carbohydrates | 糖原和核酸 Glycogen and nucleic acids | 1063.47 | 1063.62 | 1060.50 |
| 磷酸二酯>PO2中P=O的不对称伸缩振动峰 P=O str (asym) of>PO2 phosphodiesters | 主要是核酸 Mainly nucleic acids | 1244.96 | 1239.16 | 1236.03 |
| COO中C=O对称伸缩振动峰 C=O str (sym) of COO | 氨基酸侧链,脂肪酸 Amino acid side chains, fatty acids | 1393.72 | 1393.87 | 1393.75 |
| 酰胺II中蛋白质N-H弯曲振动峰和C-N拉伸振动峰 Amide II (protein N-H bend, C-N stretch) | α螺旋 α helices | 1533.55 | 1539.65 | 1539.50 |
| 酰胺I的β-折叠结构Amide I of β-pleated sheet structures | β-折叠 β-pleated sheet | 1640.66 | 1634.86 | 1634.71 |
| >CH2中C-H不对称伸缩振动峰 C-H str (asym) of>CH2 | 主要是脂类Mainly lipids | 2931.90 | 2926.08 | 2925.95 |
| 羟基O-H伸缩振动峰O-H str of hydroxyl groups | 多糖和蛋白质Polysaccharides, proteins | 3288.93 | 3283.10 | 3274.05 |
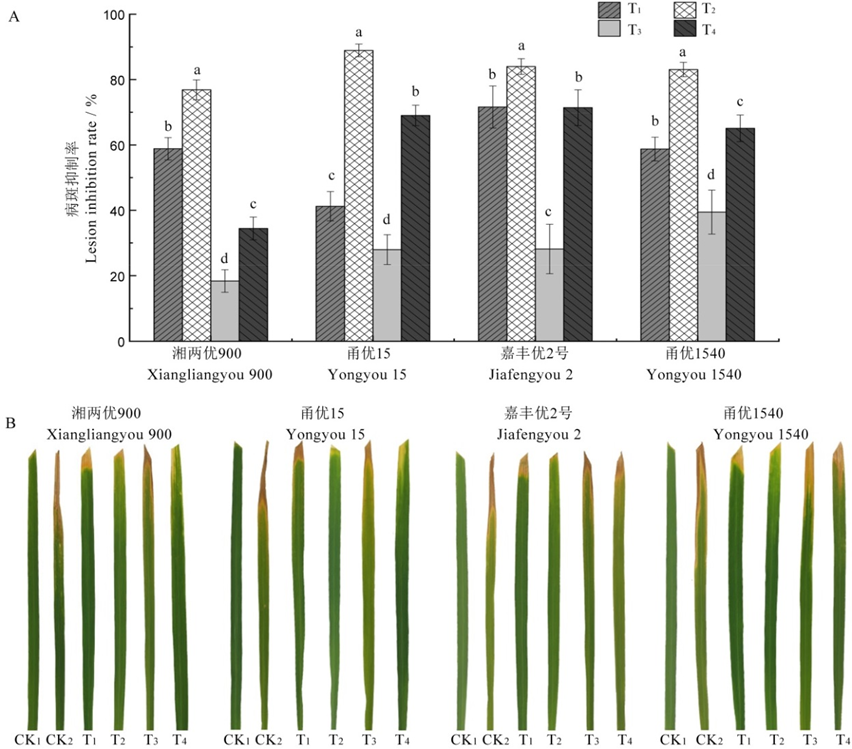
图8 4个品种水稻的病斑抑制率和防治效果 CK1?无菌水阴性对照;CK2?Xooc阳性对照;T1?发酵液治疗处理;T2?发酵液预防处理;T3?发酵滤液治疗处理;T4?发酵滤液预防处理。不同小写字母表示处理间差异显著,P<0.05。
Fig. 8. Inhibition rate and control effect of four rice varieties. CK1, Sterile water negative control; CK2, Xooc positive control; T1, Fermentation liquid cure treatment; T2, Fermentation liquid prevention treatment; T3, Fermentation filtrate cure treatment; T4, Fermentation filtrate prevention treatment. Different lowercase letters indicate significant difference at P<0.05.
| 处理 Treatment | 病情指数 Disease index | 相对防效 Relative control effect / % | ||||||
|---|---|---|---|---|---|---|---|---|
| 湘两优900 Xiangliangyou 900 | 甬优15 Yongyou 15 | 嘉丰优2号 Jiafengyou 2 | 甬优1540 Yongyou 1540 | 湘两优900 Xiangliangyou 900 | 甬优15 Yongyou 15 | 嘉丰优2号 Jiafengyou 2 | 甬优1540 Yongyou 1540 | |
| CK1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CK2 | 60.00 e | 64.00 e | 48.67 d | 54.00 e | 0 | 0 | 0 | 0 |
| T1 | 29.33 b | 39.33 c | 18.67 b | 24.00 b | 51.11 c | 38.54 b | 61.65 b | 55.56 b |
| T2 | 19.33 a | 10.00 a | 10.00 a | 10.00 a | 67.78 d | 84.38 d | 79.45 c | 81.48 d |
| T3 | 50.00 d | 46.00 d | 36.00 c | 32.67 d | 16.67 a | 28.13 a | 26.03 a | 39.51 a |
| T4 | 40.00 c | 22.00 b | 18.67 b | 23.33 c | 33.33 b | 65.63 c | 61.65 b | 56.79 c |
表4 4个品种水稻的病情指数及相对防治效果
Table 4. Disease index and relative control effect of four rice varieties.
| 处理 Treatment | 病情指数 Disease index | 相对防效 Relative control effect / % | ||||||
|---|---|---|---|---|---|---|---|---|
| 湘两优900 Xiangliangyou 900 | 甬优15 Yongyou 15 | 嘉丰优2号 Jiafengyou 2 | 甬优1540 Yongyou 1540 | 湘两优900 Xiangliangyou 900 | 甬优15 Yongyou 15 | 嘉丰优2号 Jiafengyou 2 | 甬优1540 Yongyou 1540 | |
| CK1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CK2 | 60.00 e | 64.00 e | 48.67 d | 54.00 e | 0 | 0 | 0 | 0 |
| T1 | 29.33 b | 39.33 c | 18.67 b | 24.00 b | 51.11 c | 38.54 b | 61.65 b | 55.56 b |
| T2 | 19.33 a | 10.00 a | 10.00 a | 10.00 a | 67.78 d | 84.38 d | 79.45 c | 81.48 d |
| T3 | 50.00 d | 46.00 d | 36.00 c | 32.67 d | 16.67 a | 28.13 a | 26.03 a | 39.51 a |
| T4 | 40.00 c | 22.00 b | 18.67 b | 23.33 c | 33.33 b | 65.63 c | 61.65 b | 56.79 c |
| [1] | 张洪程, 胡雅杰, 杨建昌, 戴其根, 霍中洋, 许轲, 魏海燕, 高辉, 郭保卫, 邢志鹏, 胡群. 中国特色水稻栽培学发展与展望[J]. 中国农业科学, 2021, 54(7): 1301-1321. |
| Zhang H C, Hu Y J, Yang J C, Dai Q G, Huo Z Y, Xu K, Wei H Y, Gao H, Guo B W, Xing Z P, Hu Q. Development and prospect of rice cultivation in China[J]. Scientia Agricultura Sinica, 2021, 54(7): 1301-1321. (in Chinese with English abstract) | |
| [2] | 亓璐, 张涛, 曾娟, 李春广, 李天娇, 赵艳丽, 闫硕. 近年我国水稻五大产区主要病害发生情况分析[J]. 中国植保导刊, 2021, 41(4): 37-42. |
| Qi L, Zhang T, Zeng J, Li C G, Li T J, Zhao Y L, Yan S. Analysis of the occurrence and control of diseases in five major rice-producing areas in China in recent years[J]. China Plant Protection, 2021, 41(4): 37-42. (in Chinese with English abstract) | |
| [3] | 杨雪, 徐会永, 臧昊昱, 冯晓霞, 潘锐, 谷春艳, 高同春. 水稻病害防控现状及对策建议[J]. 现代农药, 2022, 21(3): 1-5. |
| Yang X, Xu H Y, Zang H Y, Feng X X, Pan R, Gu C Y, Gao T C. Current situation and countermeasures of rice disease control[J]. Modern Agrochemicals, 2022, 21(3): 1-5. (in Chinese with English abstract) | |
| [4] | 张鑫, 邢立志. 我国水稻主要病害防治研究进展[J]. 安徽农业科学, 2019, 47(23): 11-13. |
| Zhang X, Xing L Z. Research progress of main disease prevention of rice in China[J]. Journal of Anhui Agricultural Sciences, 2019, 47(23): 11-13. (in Chinese with English abstract) | |
| [5] | 张荣胜, 陈志谊, 刘永锋. 水稻细菌性条斑病研究进展[J]. 江苏农业学报, 2014, 30(4): 901-908. |
| Zhang R S, Chen Z Y, Liu Y F. Advances in rice bacterial leaf streak researches[J]. Jiangsu Journal of Agricultural Sciences, 2014, 30(4): 901-908. (in Chinese with English abstract) | |
| [6] | 周蒙. 中国生物农药发展的现实挑战与对策分析[J]. 中国生物防治学报, 2021, 37(1): 184-192. |
| Zhou M. The realistic challenge and countermeasure analysis of the development of biological pesticide in China[J]. Chinese Journal of Biological Control, 2021, 37(1): 184-192. (in Chinese with English abstract) | |
| [7] | 方园, 彭勇政, 廖长贵, 陈路生, 周琦, 黄俭, 阎依超, 王慕媛, 张祎坤, 邹丽芳, 陈功友. 一株具有防病促生功能的贝莱斯芽孢杆菌SF327[J/OL]. 微生物学报, 2022, 62(10): 4071-4088. |
| Fang Y, Peng Y Z, Liao C G, Chen L S, Zhou Q, Huang J, Yan Y C, Wang M Y, Zhang Y K, Zou L F, Chen G Y. Bacillus velezensis SF327, a potential biocontrol agent with the functions of preventing plant diseases and promoting plant growth[J/OL]. Acta Microbiologica Sinica, 2022, 62(10): 4071-4088. (in Chinese with English abstract) | |
| [8] | 黄梦桑, 杨瑞环, 阎依超, 李逸朗, 方园, 周琦, 陈功友, 邹丽芳. 一株具有防治水稻条斑病潜力的高地芽胞杆菌181-7[J]. 植物病理学报, 2021, 51(6): 962-974. |
| Huang M S, Yang R H, Yan Y C, Li Y L, Fang Y, Zhou Q, Chen G Y, Zou L F. Bacillus altitudinis 181-7, a potential biocontrol agent against bacterial leaf streak of rice[J]. Acta Phytopathologica Sinica, 2021, 51(6): 962-974. (in Chinese with English abstract) | |
| [9] | Hata E M, Yusof M T, Zulperi D. Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8[J]. The Plant Pathology Journal, 2021, 37(2): 173-181. |
| [10] | 魏赛金. 有益微生物在水稻病害防治的研究进展与应用现状[J]. 生物灾害科学, 2020, 43(1): 1-7. |
| Wei S J. Advance and status in the application of beneficial microorganisms in the control of rice diseases[J]. Biological Disaster Science, 2020, 43(1): 1-7. (in Chinese with English abstract) | |
| [11] | 胡凌鸣, 徐春毅, 罗嘉琪, 张婧婧, 陆洁俐, 何智鹏, 蒋冬花. 一株拮抗水稻白叶枯病菌的淡紫灰链霉菌的筛选鉴定及其生防效果研究[J]. 植物病理学报, 2022, 52(2): 223-234. |
| Hu L M, Xu C Y, Luo J Q, Zhang J J, Lu J L, He Z P, Jiang D H. Screening, identification and biocontrol effect of a strain of Streptomyces lavendulae against Xanthomonas oryzae pv. oryzae[J]. Acta Phytopathologica Sinica, 2022, 52(2): 223-234. (in Chinese with English abstract) | |
| [12] | Shi T T, Guo X, Zhu J L, Hu L M, He Z P, Jiang D H. Inhibitory effects of carbazomycin B produced by Streptomyces roseoverticillatus 63 against Xanthomonas oryzae pv. oryzae[J]. Frontiers in Microbiology, 2021, 12: 616937. |
| [13] | Selim M S M, Abdelhamid S A, Mohamed S S. Secondary metabolites and biodiversity of actinomycetes[J]. Journal of Genetic Engineering and Biotechnology, 2021, 19(1): 72. |
| [14] |
Jiang B, Wang Z Y, Xu C X, Liu W J, Jiang D H. Screening and identification of Aspergillus activity against Xanthomonas oryzae pv. oryzae and analysis of antimicrobial components[J]. Journal of Microbiology, 2019, 57: 597-605.
PMID |
| [15] | Yang R, Li S, Li Y, Yan Y, Fang Y, Zou L, Chen G. Bactericidal effect of Pseudomonas oryziphila sp. nov., a novel Pseudomonas species against Xanthomonas oryzae reduces disease severity of bacterial leaf streak of rice[J]. Frontiers in Microbiology, 2021, 12: 759536. |
| [16] | Li W, Long Y, Mo F, Shu R, Yin X, Wu X, Zhang R, Zhang Z, He L, Chen T, Chen J. Antifungal activity and biocontrol mechanism of Fusicolla violacea J-1 against soft rot in kiwifruit caused by Alternaria alternata[J]. Journal of Fungi, 2021, 7(11): 937. |
| [17] |
Bruisson S, Zufferey M, L'Haridon F, Trutmann E, Anand A, Dutartre A, De Vrieze M, Weisskopf L. Endophytes and epiphytes from the grapevine leaf microbiome as potential biocontrol agents against phytopathogens[J]. Frontiers in Microbiology, 2019, 10: 2726.
PMID |
| [18] | 曾益波, 刘骏, 赵国盛, 吴希阳, 陈岳, 苏品, 张德咏, 刘勇. 光合细菌PSB06浸种对水稻促生作用研究[J]. 杂交水稻, 2018, 33(3): 50-53. |
| Zeng Y B, Liu J, Zhao G S, Wu X Y, Chen Y, Su P, Zhang D Y, Liu Y. Promoting effects of soaking seed with photosynthetic bacterium PSB06 on rice growth[J]. Hybrid Rice, 2018, 33(3): 50-53. (in Chinese with English abstract) | |
| [19] | Cai X, Wang X, Chen Y, Wang Y, Song D, Gu Q. A natural biopreservative: Antibacterial action and mechanisms of Chinese Litsea mollis Hemsl. extract against Escherichia coli DH5α and Salmonella spp[J]. Journal of Dairy Science, 2019, 102(11): 9663-9673. |
| [20] | Wu L, Wu H, Chen L, Yu X, Borriss R, Gao X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens[J]. Scientific Reports, 2015, 5: 12975. |
| [21] |
Jasrotia S, Salgotra R K, Sharma M. Efficacy of bioinoculants to control of bacterial and fungal diseases of rice (Oryza sativa L.) in northwestern Himalaya[J]. Brazilian Journal of Microbiology, 2021, 52(2): 687-704.
PMID |
| [22] |
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney R K. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea[J]. Brazilian Journal of Microbiology, 2016, 47(1): 85-95.
PMID |
| [23] | Rais A, Jabeen Z, Shair F, Hafeez F Y, Hassan M N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae[J]. PLoS One, 2017, 12(11): e0187412. |
| [24] |
Suárez-Moreno Z R, Vinchira-Villarraga D M, Vergara-Morales D I, Castellanos L, Ramos F A, Guarnaccia C, Degrassi G, Venturi V, Moreno-Sarmiento N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens[J]. Frontiers in Microbiology, 2019, 10: 290.
PMID |
| [25] | Vurukonda SSKP, Giovanardi D, Stefani E. Plant growth promoting and biocontrol activity of Streptomyces spp. as Endophytes[J]. International Journal of Molecular Sciences, 2018, 19(4): 952. |
| [26] |
Olanrewaju O S, Babalola O O. Streptomyces: implications and interactions in plant growth promotion[J]. Applied Microbiology and Biotechnology, 2019, 103(3): 1179-1188.
PMID |
| [27] | 项鹏, 李宝华, 张武, 鹿文成, 李红鹏. 一株具诱导大豆抗大豆胞囊线虫的沙阿霉素链霉菌及应用: CN108004183A[P]. 2018-05-08. |
| Xiang P, Li B H, Zhang W, Lu W C, Li H P. A strain of Streptomyces zaomyceticus capable of inducing soybean resistance to soybean cyst nematode and its application: CN108004183A[P]. 2018-05-08. (in Chinese with English abstract) | |
| [28] |
Kudoyarova G, Arkhipova T, Korshunova T, Bakaeva M, Loginov O, Dodd I C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses[J]. Frontiers in Plant Science, 2019, 10: 1368.
PMID |
| [29] | Park Y G, Mun B G, Kang S M, Hussain A, Shahzad R, Seo C W, Kim A Y, Lee S U, Oh K Y, Lee D Y, Lee I J, Yun B W. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones[J]. PLoS One, 2017, 12(3): e0173203. |
| [30] |
Lee K E, Radhakrishnan R, Kang S M, You Y H, Joo G J, Lee I J, Ko J H, Kim J H. Enterococcus faecium LKE12 cell-free extract accelerates host plant growth via gibberellin and indole-3-acetic acid secretion[J]. Journal of Microbiology and Biotechnology, 2015, 25(9): 1467-75.
PMID |
| [31] |
Compant S, Samad A, Faist H, Sessitsch A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application[J]. Journal of Advanced Research, 2019, 19: 29-37.
PMID |
| [1] | 冯爱卿, 汪聪颖, 苏菁, 封金奇, 陈凯玲, 林晓鹏, 陈炳, 梁美玲, 杨健源, 朱小源, 陈深. 水稻细菌性条斑病抗性新品系的创制及其农艺性状分析[J]. 中国水稻科学, 2023, 37(6): 587-596. |
| [2] | 谢关林,孙漱沅,王公金,朱献玳,陈军昂,叶彦弧,冯忠民,梁梅新. 水稻细菌性条斑病种子带菌检测技术研究:I. 免疫放射分析法[J]. 中国水稻科学, 1990, 4(3): 127-132 . |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||